The following sections discuss the existing evidence linking comorbidities to COVID-19 outcomes during each of these distinct phases of COVID-19.
Comorbidities, multimorbidity and COVID-19
Abstract
The influence of comorbidities on COVID-19 outcomes has been recognized since the earliest days of the pandemic. But establishing causality and determining underlying mechanisms and clinical implications has been challenging—owing to the multitude of confounding factors and patient variability. Several distinct pathological mechanisms, not active in every patient, determine health outcomes in the three different phases of COVID-19—from the initial viral replication phase to inflammatory lung injury and post-acute sequelae. Specific comorbidities (and overall multimorbidity) can either exacerbate these pathological mechanisms or reduce the patient’s tolerance to organ injury. In this Review, we consider the impact of specific comorbidities, and overall multimorbidity, on the three mechanistically distinct phases of COVID-19, and we discuss the utility of host genetics as a route to causal inference by eliminating many sources of confounding. Continued research into the mechanisms of disease-state interactions will be crucial to inform stratification of therapeutic approaches and improve outcomes for patients.
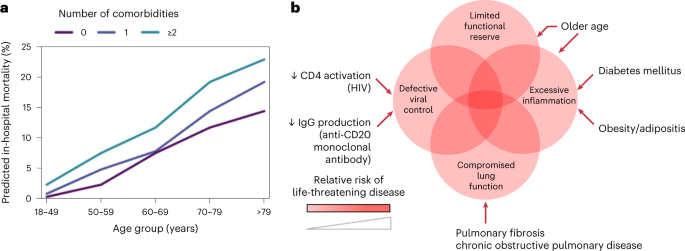
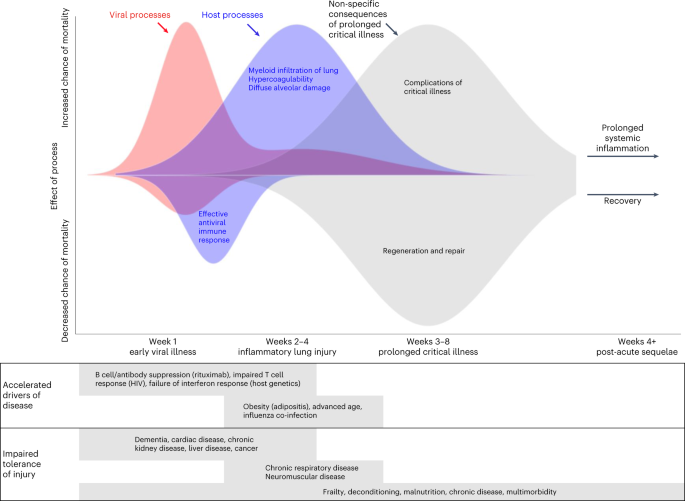
Understanding the impact of comorbidity in COVID-19 has two broad purposes—it enables prioritization for interventions such as preventive measures, vaccination and early treatment, and it may deepen understanding of the underlying biology of the disease. These principles are reflected in public-health guidance that prioritizes some groups for vaccination, and clinical guidance to use antivirals to prevent hospitalization in people with specific comorbidities. Some risk factors reveal mechanisms that either exacerbate the underlying disease processes or reduce the ability of a patient to cope with the injurious consequences of these processes. The importance of comorbidity in modifying severity and outcomes in COVID-19 was recognized in the earliest scientific reports1,2. Here, we use the term ‘comorbidity’ to refer to any long-term health condition that coexists in an individual with a specific condition of interest, in this case COVID-19 (ref. 3). This is distinct from multimorbidity, which describes the presence of two or more long-term health conditions in an individual without reference to COVID-19—and it is in itself a major, growing public-health challenge4. Modeling studies have estimated that 1.7 billion people globally (22% of the population) have at least one comorbidity that is associated with an increased risk of developing severe COVID-19 (ref. 5). The most direct evidence related to the clinical impact of any comorbidity comes from epidemiological associations with key outcome measures. In acute COVID-19, the most widely used outcomes describe disease severity with pragmatic measures, including hospitalization, requirement for oxygen treatment or organ support and mortality6. Interpretation of associations between various comorbidities and disease severity outcomes requires consideration of the distinct biological events, social factors and clinical decisions that lead to these outcomes. Although we have convincing evidence that host features (for example age, sex and genetics) are primary determinants of disease progression7,8, it is important to note that comorbidities can also affect risk of exposure: both increasing risk (for example, in outbreaks in care homes) and decreasing it (for example, through shielding behaviors). The clinical decisions to admit a patient to the hospital or to initiate organ support are also strongly influenced in complex ways by the presence of comorbidities, multimorbidity and frailty, and even an objective outcome, such as mortality, can be difficult to interpret because different sequences of biological events can lead to death. Like many other potentially life-threatening infectious diseases, the spectrum of disease severity resulting from SARS-CoV-2 infection is wide, with asymptomatic infection being the most likely outcome and life-threatening disease being extremely uncommon. Importantly, COVID-19 has three distinct phases (Box 1), which we categorize as acute viral illness, immune-mediated inflammatory lung injury, and post-acute sequelae of COVID-19 (PASC)9. Patients at each disease stage have divergent responses to therapy, reflecting different underlying biological mechanisms. In this Review, we first discuss the challenges associated with comorbidity–outcome associations, including the potential biases and confounding factors that can influence their interpretation. Next, we consider the impact of specific comorbidities and overall multimorbidity on the three mechanistically distinct phases of COVID-19. In each phase, where evidence exists, we consider the implications of associations with comorbid illness in driving the underlying pathophysiological mechanisms, or in reducing a patient’s tolerance of the consequences of those mechanisms. For example, some comorbidities worsen immune-mediated lung injury by reducing viral clearance or exacerbating inflammation, whereas other comorbidities reduce lung function at baseline, predisposing a person to respiratory failure at a level of lung injury that would be well-tolerated by someone without one of these comorbidities. Synthesizing epidemiological and host genetic signals with the current understanding of the underlying mechanisms of disease, we draw some tentative conclusions about the implications of comorbidities in COVID-19, and describe how future work can maximize the value of our existing knowledge base. Findings from large, observational studies have been used as the starting point to inform hypotheses to explore the biological mechanisms through which comorbidities predispose individuals to severe COVID-19. However, caution needs to be exercised before inferring causality from these associations. Spurious or distorted associations can arise for several reasons that are related to study design and sampling, and interpreting putative causal relationships between specific comorbidities and acute COVID-19 is difficult owng to complex relationships with outcomes. Associations reported between comorbidities and outcomes in studies of populations of hospitalized patients with COVID-19 could be biased owing to the criteria for entry into the study (that is, requirement for hospitalization) being causally associated with both the comorbidity (collider bias) and the outcome10. Studies undertaken in populations that are not restricted to hospitalized individuals are less likely to report associations affected by this bias. However, comorbidity–outcome associations can be distorted if sampling does not require hospitalization, including in cohorts sampled from general populations, because the risk of developing the outcome (usually severe COVID-19 disease) is a combination of the probabilities of contracting the virus, seeking testing, and developing severe disease. Studies that use a positive PCR test as part of the case definition usually rely on non-random testing, which could be biased by associations between testing patterns and features related to comorbidity status and/or severity (for example, hospitalization or severe symptoms). Similarly, the likelihood of being exposed to the virus might differ by comorbidity status, particularly if non-pharmacological interventions, such as shielding or compliance with mask wearing, are more common in those with comorbidities. Those with comorbidities might also be more cautious about social interactions, even when public-health authorities no longer advise non-pharmacological measures11. Even when a comorbidity causally influences outcomes, the underlying mechanisms might not be specific to COVID-19. Comorbidities can predispose an individual to hospitalization, and even intensive-care unit (ICU) admission, as a result of almost any acute illness, a non-specific concept that is widely understood among clinicians, but often impossible to quantify in biological terms. Specific examples include lung conditions, such as chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis, which can be measured by an objective reduction in lung function tests, and chronic kidney disease, which is quantified by reductions in glomerular filtration rate. These measurable reductions in the functional capacity of specific organs magnify the proportional impact of a new insult—there is less function to lose before the organ fails to meet the minimum requirements for survival. Frailty, malnourishment, and chronic illness without organ dysfunction can impair an individual’s capacity to tolerate even a relatively minor physiological stressor, resulting in an increased likelihood of hospitalization and/or the need for organ support. Caution is also needed when severity is defined by health-service use or intervention (that is, hospitalization, critical-care admission, or respiratory support), as the presence of a comorbidity might influence clinical decision making, either lowering or increasing the threshold for hospitalization or provision of organ support. This is not specific to clinical management of COVID-19: in a multicenter, Europe-wide study of patients in the ICU with acute respiratory distress syndrome, those with a comorbidity were less likely to receive invasive mechanical ventilation and other interventions for severe hypoxaemia than those without a comorbidity12. It is possible that the extreme capacity strain in many areas during the peaks of the COVID-19 pandemic may have augmented this effect. When the effectiveness of an intervention does not rely on mechanisms specific to the comorbidity–outcome relationship, comorbidities can simply act as prognostic markers of poor outcomes. Because the best evidence suggests that common, severe comorbidities do not have markedly differing effects on risk of death13, the widely used and validated 4C score for COVID-19 mortality risk, an early output from the ISARIC4C study7, does not separate individual comorbidities, using only the total number of comorbid illnesses present (Fig. 1). This pattern is consistent with other studies on the impact of comorbidity on risk of acute disease. However, some specific comorbidities merit consideration in COVID-19 owing to their potential mechanistic implications (discussed below). a, To illustrate the impact of multimorbidity, the ISARIC4C mortality score was used to predict in-hospital mortality for hypothetical male patients in different age groups and with different numbers of comorbidities, assuming other variables used in the score remained the same (respiratory rate < 20 breaths min−1; peripheral oxygen saturation > 91%; Glasgow Coma Scale score 15; urea < 7 mmol l−1; C-reactive protein < 50 mg l−1). The graph illustrates the increasing risk of mortality with increasing age and increasing number of comorbidities, and that comorbidity count has an additive effect to age. b, Additive effects (indicated by color shading) exerted by comorbidities (multimorbidity), contributing to disease severity through different potential mechanisms. One highly effective method to test causal mechanistic hypotheses is Mendelian randomization (Box 2), which uses the random genetic variation across the population to draw an inference about the causal link between one biological observation and another. COVID-19 was initially described as a biphasic illness15, by a Chinese group using the ISARIC Clinical Characterisation Protocol16—and this quickly became familiar to clinicians worldwide. It was noted that death could occur by two different routes, as both the initial viral illness (associated with high viral replication in the upper respiratory tract) and the later inflammatory lung injury phase can cause life-threatening disease17,18,19. The strongest evidence for mechanistic differences between the acute viral illness and the inflammatory lung injury phases of COVID-19 comes from the RECOVERY trial results for dexamethasone20. This study was remarkable for its speed and the scale of its impact, but also because it is a rare example of a major change in clinical practice due to a subgroup analysis in a single trial. The results showed a net benefit overall, but a prespecified subgroup analysis indicated that there was a greater reduction in mortality among patients who required invasive ventilation, and a trend towards harm among patients who did not require oxygen, at the time of randomization. Just as the impact of this treatment differs between hypoxic and nonhypoxic COVID-19, the impact of some comorbidities is also likely to differ between the two disease phases. Among patients who require prolonged invasive ventilation, there is an accumulating risk over time of complications associated with organ support and prolonged critical illness (Fig. 2). These include critical illness neuropathy and myopathy21, secondary infections including ventilator-associated pneumonia, and delirium. These problems are not unique to patients with COVID-19 but are an important mechanism by which comorbid illnesses can significantly increase mortality by reducing tolerance of injury. The progress of a range of biological processes are plotted over time, according to their hypothetical impact on the probability of survival. Those initiated by the virus (for example, replication, production of virulence factors, and inhibition of host interferon response) are shown in red; those initiated by the host (for example, initial antibody response and cell-mediated immunity preventing viral replication, and later innate immune-mediated pulmonary inflammation) are shown in blue. The lower panels provide examples of comorbidities contributing to enhanced disease pathogenesis or impaired tolerance at each stage. Although most people infected with SARS-CoV-2 fully recover, a substantial minority develops persisting symptoms—either due to the virus itself, a non-resolving host inflammatory response, or to non-specific effects of critical illness—leading to the concept of COVID-19 as a tri-phasic illness. In this section, we discuss comorbid illnesses likely to have their greatest impact during the initial phase of disease, when viral replication is greatest. We do not suggest that this phase and subsequent phases are independent—indeed, genetic evidence suggests that people with impaired control of viral replication, for example owing to defective type I interferon signaling, are also predisposed to lung inflammation22. A growing body of evidence from observational studies and clinical trials of antivirals suggests that lung inflammation is to some extent proportional to, and primed by, viral load—and is not an idiosyncratic immune response23. Hence, the disease associations discussed here are also likely to predispose a patient to inflammatory lung injury. In order to synthesize a mechanistic understanding of pathogenesis with the observed associations with clinical outcomes, we draw a distinction between comorbidities that are expected to affect either resistance to viral infection or tolerance of the consequences. We use the term resistance to describe the ability of the host to control pathogen replication, whereas the term ‘tolerance’ in the context of this Review describes the ability of a patient to cope with a given degree of injury (whether pathogen- or host-mediated). Studies restricted to patients hospitalized with COVID-19 demonstrate a substantially higher prevalence of comorbidity than in the general population. In the ISARIC4C study, which recruited patients hospitalized with COVID-19 in the UK using a globally harmonized protocol16, more than three-quarters of patients had at least one comorbidity, and patients with cardiac disease, pulmonary disease, chronic kidney disease, obesity, cancer, chronic neurological disorders, dementia, and/or liver disease had an increased risk of in-hospital mortality1. Similar findings were reported for a more limited set of comorbidities in early reports from the Chinese mainland, albeit these were limited by sample size2. The need to rapidly mobilize data collection on affected patients necessitated the limitation of these early studies to hospitalized populations, comprising a mixture of patients requiring hospital care owing to acute viral illness and patients hospitalized because of inflammatory lung disease. In a wider cohort study using primary-care data from 40% of the English population24, 15 comorbidity groupings were evaluated together with body-mass index (BMI). The most common comorbidity was hypertension (34.3%), followed by asthma (15.9%) and diabetes (9.9%). In age- and sex-adjusted regression models, all comorbidity groups were associated with increased risk of death from COVID-19; the greatest risk was found in organ-transplant recipients (hazard ratio (HR) 6.00 (95% confidence interval (CI) 4.73–7.61)) and in those with chronic kidney disease (HR 3.48 (3.23–3.75)). For most comorbidity groupings, the magnitude of association was greater in analyses restricted to the earlier pandemic period, shortly after social-distancing policies were introduced, and vulnerable groups were advised to minimize face-to-face contact with others (shielding). This highlights the need to interpret associations in the context of wider societal determinants that modify exposure to the virus11 Specific immune effector mechanisms required for effective resistance vary by pathogen. For example, people with neutropenia are at specific risk of invasive infection with gram-negative bacilli compared with people with normal neutrophil function25. By contrast, because Staphylococcus aureus persists within neutrophils that disseminate it to distant sites through the bloodstream, S. aureus disease is under-represented in people with neutropenia26. ‘Immunosuppression’ as a broad term is therefore unhelpful in understanding the pathogenesis of infectious agents, but specific examples contribute to the identification of key effectors of resistance. SARS-CoV-2 replication occurs in the upper respiratory tract, where expression of ACE2 (the receptor that mediates SARS-CoV-2 viral entry) is highest, and viral load at this site is positively correlated with disease severity27,28,29. Although organ-transplant recipients are one of the groups at greatest risk of death from COVID-19 (ref. 24), an analysis of a large US COVID-19 database (n = 222,575) found that immunosuppressive therapy before hospitalization (for any reason, including transplant) was not associated with in-hospital mortality30. However, when individual drug classes were considered, increased mortality was seen specifically for the B cell-depleting anti-CD20 monoclonal antibody rituximab. In 422 people with COVID-19 who had recently received anti-CD20 therapy (330 of whom had received rituximab) for comorbid auto-inflammatory diseases, in-hospital mortality was associated with more recent (<6 months) receipt of the drug, and 61% of infected people had received their most recent dose within 3 months31. In this study, anti-CD20 treatment was also associated with prolonged upper respiratory tract viral shedding and relapses. Seroconversion was associated with having received anti-CD20 therapy further in the past and with a lower likelihood of virologic recurrence. The consequences of infection with human immunodeficiency virus (HIV) identify another component of the adaptive immune system that contributes to resistance to SARS-CoV-2. People living with HIV who are hospitalized because of COVID-19 are usually younger than those without HIV who are hospitalized because of the disease, and there is higher in-hospital mortality in this group32. In hospitalized people with HIV and COVID-19, a CD4 T cell count of fewer than 350 cells μl−1 was independently associated with severe disease33. In a cohort of people with HIV who had been receiving antiretroviral therapy for more than 2 years, with an undetectable viral load and CD4 T cell counts of 133–1,360 cells μl−1, a positive relationship was found between the CD4:CD8 ratio and the extent of T cell activation against SARS-CoV-2 (ref. 34). Therefore, despite virologic suppression and apparent immune reconstitution, subtle defects that compromise T cell responses likely remain in people with HIV. The implications of B cell-depleting immunosuppressive therapies and HIV-associated T cell impairment on COVID-19 pathogenesis identify these immune responses as crucial for COVID-19 resistance, consistent with observations from human immunology studies of respiratory syncytial virus35. Other comorbidities associated with altered immune function could provide further mechanistic information about resistance to SARS-CoV-2, but require further investigation. Cytomegalovirus (CMV) is a ubiquitous herpesvirus that latently infects most people. Reactivation can occur in the context of lymphoid immunosuppression, including advanced HIV infection, critical illness, or immunosuppressive therapy. Although reactivation can lead to end organ disease (for example, pneumonitis), latent infection is also associated with immunological perturbation and potentially immunosenescence36. As expected, CMV reactivation has been documented in severe COVID-19 (refs. 37,38). However, specific sampling of the respiratory tract indicated that latent CMV infection is associated with both increased likelihood of COVID-19 and increased disease severity, even in the absence of virologically confirmed CMV reactivation39. This association was replicated in a second cohort, where an adjusted analysis demonstrated that the risk was independent of age and other comorbidities, and that CMV seropositivity was most strongly associated with severe disease in people younger than 60 (ref. 40). T cell alterations—specifically activation of effector memory T cells expressing CD45RA—in CMV-seropositive people with COVID-19 are similar to those in seropositive people without COVID-19 (ref. 39). Any immunological effects of latent CMV on COVID-19 pathogenesis are therefore likely to be distinct from classical T cell consequences. In COVID-19, recent work has suggested that variants that affect the key viral-entry mechanisms for SARS-CoV-2 in human cells (ACE2 and TMPRSS2 proteins) alter the probability of the mutant causing severe disease41. However, ACE2 is also involved in blood pressure homeostasis, and ACE inhibitors, used as a first-line approach to managing hypertension, are associated with increased ACE2 expression in some organs42. Multiple studies early in the pandemic reported an increased risk of mortality in people with hypertension, leading to the biologically plausible hypothesis that this was mediated through increased expression of the viral receptor ACE2 (although this has now been disproven43). The epidemiologic association between hypertension and COVID-19 has not been confirmed in larger more robust studies (reviewed in ref. 44). Furthermore, ACE2 expression in alveolar pneumocytes is actually infrequent, and virus arrives in the distal lung after uptake by migrating macrophages29. These macrophages take up virus particles by endocytosis and no productive replication occurs, but this is associated with macrophage activation, engagement of a pro-inflammatory programme, and associated alveolar injury45. Therefore, although superficially plausible, there is no definitive link between ACE2 expression and lung pathology in COVID-19. The putative association with hypertension has not been consistently reproduced, and ACE2 inhibition does not upregulate ACE2 protein expression by airway epithelial cells. ACE2 expression is, however, upregulated in people with chronic obstructive pulmonary disease46, and this has been confirmed at the protein level in respiratory samples47. Nevertheless, in our view, it is more likely that a reduced threshold for respiratory failure (rather than ACE2 expression) underlies the association of COPD with mortality in those with COVID-19. More broadly, this highlights the implications of frailty and multimorbidity on COVID-19 pathogenesis. The significant association, in multiple studies, of a very broad range of comorbidities with hospitalization and death, is consistent across not only studies of COVID-19, but also a range of other respiratory and systemic illnesses. This, together with the strong, consistent signal that multimorbidity is the biggest risk factor for severe COVID-19, suggests that the impact of these comorbidities is not specific to COVID-19, or even to respiratory disease. Patients with severe underlying chronic disease often maintain a precarious level of function, in which an acute pyrexial illness may precipitate decompensation of the underlying disease. This is consistent with an observational study of pre-morbid ECOG (Eastern Cooperative Oncology Group) performance status on outcomes of acute COVID-19. After correcting for relevant physiologic variables, performance status (which was positively correlated with the number of comorbidities) remained independently associated with in-patient mortality, with an effect size greater than age. Similar findings have been reported using the clinical frailty score48. This leads us to conclude that the major impact of most comorbidities in the acute viral illness phase is a generic consequence of frailty and reduced physiological reserve. The general effects of comorbidities and/or frailty are also expected to reduce tolerance of hypoxaemic respiratory failure, a clinical consequence of inflammatory lung injury. Some comorbid illnesses are thought to have specific effects that either drive the underlying disease process (for example, adipositis in obesity) or specifically reduce tolerance of respiratory failure (for example, chronic respiratory disease and neuromuscular disease; Fig. 1b). For comorbidities that have been studied intensively using human genetics, Mendelian randomization offers an opportunity to test the hypothesis that each comorbidity directly causes severe or fatal outcomes in COVID-19. One such comorbidity—obesity—has been consistently found to be associated with critical COVID-19 in Mendelian randomization studies49. Although Mendelian randomization is a robust and powerful tool for inferring causal relationships between mediators and outcomes in COVID-19, the reliance of the GenOMICC study on opportunistic population control groups (that is, UK Biobank, Generation Scotland and 100,000 Genomes projects) could inflate the effect of genes associated with obesity, because population genetic studies are enriched for patients with lower BMIs50. This is unlikely to affect the primary genome-wide significant findings of the study because they are robust to sensitivity analyses controlling stringently for BMI. However, these Mendelian randomization studies rely on signals from weaker genetic associations, which have not been confirmed in the same way. Most of these limitations apply equally to the many other comorbidities associated with poor outcomes in COVID-19, so it is of interest that the effect of obesity and adiposity is both consistent49 and that similar effects have not been observed for other common, related conditions49. Since the genetic signals underlying this observation arise primarily from a study among only critically ill patients51, we think this effect is likely mediated through pneumonitis, rather than frailty. One plausible mechanism through which obesity might influence the development of pneumonitis is low-grade systemic innate immune inflammation (adipositis), which could predispose the infected organ to innate immune injury when viral replication is not contained in the early phase of the illness. We have previously proposed a similar mechanism in life-threatening influenza52. Although it was not supported by a strong Mendelian randomization signal, an association was identified early in the pandemic between diabetes and life-threatening disease in hospitalized people with COVID-19 (ref. 1). Owing to shared risk factors, type 2 diabetes (T2D) often co-occurs with obesity and cardiovascular disease, and is more prevalent in older individuals. In an attempt to disentangle this, an analysis using data from a nationwide diabetes registry in Sweden and matched population controls found that T2D (n = 385,021) remained an independent risk factor for both hospitalization and ICU admission in patients with COVID-19, after adjustment for age and comorbidities53. Although this study did not identify type 1 diabetes (T1D) itself as an independent risk factor (n = 44,478 with T1D), poor glycemic control in the context of T1D was associated with these outcomes. In the Swedish cohort, BMI was one of the strongest predictors of adverse outcomes in patients with T2D and COVID-19. Glycemic control in T2D, assessed by glycated hemoglobin (HbA1c), is strongly associated with obesity54. In a US cohort of people with T2D and COVID-19, poor glycemic control (determined by HbA1c levels) was associated with hospitalization, ICU admission, and invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO)55. Given the relationship between T2D, glycemic control, and obesity, Mendelian-randomization approaches have been applied to differentiate association from causation in COVID-19 severity. T2D itself has consistently been found not to be causally associated with adverse outcomes in COVID-19, in contrast to the closely related trait of obesity56,57. People with diabetes have increased susceptibility to bacterial infections, especially skin and soft-tissue infections and invasive infections involving Gram-negative bacteria. Hyperglycemia can act directly as a pro-bacterial factor in the pathogenesis of skin and soft-tissue infections58, and a hyper-inflammatory response involving interleukin-1β (IL-1β) and IL-6 is implicated in severe Gram-negative infections59. Innate immune activation driven by IL-6 and associated with high levels of IL-1β is also associated with disease severity and has been implicated causally in fatal COVID-19 (refs. 20,60,61). Mechanistic studies have provided a link between diabetes and regulation of inflammation in COVID-19, especially involving monocytes and macrophages, which are likely to be important cell types driving immunopathology62,63. In COVID-19, T2D is associated with peripheral blood monocyte activation and a transition to a CD14lo non-classical phenotype, along with increased expression of IL-6 and CCL2, indicative of a hyper-inflammatory phenotype64. Importantly, patients with T2D in this study64 also had a higher median BMI than those in the non-diabetic group (28 (interquartile range 24–32) versus 22 (21–22)), so these cellular phenotypes may be consequences of obesity, rather than T2D. Although not strictly a comorbidity, the strong effect of co-infection with influenza on risk of invasive ventilation and death in the ISARIC4C study was not seen with co-infection with other respiratory viruses65, suggesting that common mechanisms underlying lung injury might be shared between COVID-19 and influenza. Importantly, the apparently strong impact of pregnancy on risk of severe influenza52 is not apparent in COVID-19. Most strikingly of all, the risk of severe illness among infants and young children is much higher in influenza66 than in COVID-19 (ref. 67). However, the effect of old age is very striking in both conditions—in COVID-19, the risk of death is 11-fold greater among patients >80 years old than for those <50 years old1. A process with many similarities to adipositis, referred to as inflammaging—in which subclinical systemic inflammation exists, mediated in part by monocyte/macrophage activation by cell debris68—may predispose older patients with COVID-19 to lung damage mediated by the innate immune system. The interactions between inflammatory lung injury in COVID-19, adipositis, inflammaging, diabetes, and co-infections provide some important clues about the underlying molecular mechanisms of organ injury in all of these conditions. Ongoing mechanistic work in each of these conditions will continue to deepen our understanding of COVID-19, even in a future post-pandemic era when direct study of COVID-19 cases is no longer possible. Those who have survived critical illness from any cause, including non-infectious causes, have significant impairments in mental and physical health and health-related quality of life, and they experience broader socioeconomic impacts and effects on family mental health, referred to as post-intensive-care syndrome69,70,71. People who have been to the ICU are at increased risk of emergency rehospitalization for a sustained period after their episode of critical illness, compared with non-critically-ill hospitalized patients72. Those with comorbidities before critical illness are less likely to return to baseline physical function73, and have more than double the risk of emergency rehospitalization compared with those without comorbidities74For these reasons, careful analysis is required to identify whether post-acute COVID-19 sequelae are attributable and specific to SARS-CoV-2 infection, or whether they are driven by non-specific aspects of acute illness or hospitalization, or pre-existing comorbidity. Separate from post-intensive-care syndrome, post-acute morbidity is recognized after several acute viral infections, including Dengue, severe acute respiratory syndrome (SARS), and influenza75,76,77. The World Health Organization (WHO) published a consensus case definition for post-acute sequelae of COVID-19 (PASC, also known as post-COVID condition or long COVID) more than 18 months after the start of the pandemic: “Post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.”9 The epidemiology of PASC has been comprehensively summarized elsewhere and is not discussed further here78,79. However, the specific influence of pre-existing comorbidities on PASC is becoming clearer as experience and research accrue; there is now clear evidence that people with pre-existing comorbidities are more likely to develop PASC than are those without them80. One study found that prevalence of PASC ranged from 2.8% to 5.5% in people with pre-existing health conditions, compared with 1.8% in those with no health conditions81. In non-hospitalized individuals, multimorbidity is associated with increased risk of persisting symptoms at 12 weeks following acute infection. In one study that accounted for baseline symptom burden and appropriate comparators with no evidence of SARS-CoV-2 infection, most comorbidities in a list of 80 were associated with persisting symptoms at 12 weeks82, including physical comorbidities, such as COPD (HR 1.55 (1.47–1.64)), and mental-health problems, such as anxiety (HR 1.35 (1.31–1.39)). A range of specific new diagnoses following acute COVID-19 has also been described. An investigation using the national healthcare databases of the US Department of Veterans Affairs and carefully selected control populations (with propensity score weighting to ensure balance in potential confounders) demonstrated a higher risk of incident symptoms and conditions spanning all body systems, beyond the first month of illness, in a cohort of mostly male patients83. Patients with COVID-19 were also at increased risk of developing most of these conditions compared with a historic population with seasonal influenza, increasing confidence that the findings might be attributable to SARS-CoV-2 infection specifically. New cardiovascular comorbidities have also been reported in those who survive the acute phase of COVID-19. In a large study with contemporary controls from the Veterans Affairs database, COVID-19 increased the risk of cardiovascular disease by 1.6 times, translating into an additional burden of new cardiovascular disease in 45 per 1,000 people during the 12-month follow up84. This increased risk did not vary substantially by pre-existing comorbidities, although only a limited number (including obesity, chronic kidney disease, and diabetes) were assessed. This study also provided robust evidence for a relationship between increasing severity of acute COVID-19 (defined as non-hospitalized cases, hospitalized cases, and those requiring intensive care) and increased risk of incident cardiovascular disease. The increased risk that comorbidities confer on developing both PASC and new comorbidities after acute COVID-19 has implications for policy and practice. Although vaccination is highly effective at preventing severe COVID-19 disease, early evidence indicates that it is less effective at preventing PASC. In a recently published large study, vaccination reduced the risk of PASC by only 15% after breakthrough infection, with vaccine effectiveness being marginally lower in the one comorbidity subgroup (individuals with immunosuppression) that was evaluated85. For this reason, non-pharmacological interventions will likely have an ongoing role in reducing the risk of exposure to the virus, and prioritization of vaccine booster doses for people with comorbidities will continue. Individuals with comorbidities who develop COVID-19 may require closer follow up by clinicians, to ensure appropriate management through post-acute COVID-19 services.Main
Navigating causality and confounders
Mendelian randomization has some general limitations14—most importantly, the assumption that the causal pathway between a gene and a disease outcome is mediated through a single route. This limitation is particularly important in the context of comorbidities and COVID-19 severity, because the assumption of a single causal pathway is less plausible and is untestable. Nevertheless, this approach has proved a valuable tool in the complex task of differentiating association from causality, and has formed the basis of several key studies discussed below.
Impact of comorbidities during each phase of COVID-19
Acute viral illness
Inflammatory lung injury
Recovery and post-acute COVID-19 sequelae
Multimorbidity
Most research related to the impact of long-term conditions on COVID-19 has focused on single comorbidities. However, one-third of adults globally are estimated to have two or more long-term conditions86, increasing to more than two-thirds in those aged 65 or older87. In a large study of hospitalized people with COVID-19 in the UK, crude mortality in patients with multimorbidity was more than double that of those without multimorbidity (37.2% versus 17.3%), even after adjusting for demographic factors88.
Distinguishing common clusters of comorbidities in patients with severe COVID-19 might help to identify common druggable mechanisms, as well as groups at particularly high risk of poor outcomes. Among 1,706 of 360,283 participants in the UK Biobank with severe SARS-CoV-2 infection (defined as hospitalization)89, 25.3% had multimordbidity, defined from a list of 12 comorbidities. The combination of stroke and hypertension was most prevalent, and the combination of chronic kidney disease and diabetes was associated with the highest risk of severe COVID-19 (odds ratio (OR) 4.93; 95% CI 3.36–7.22). Clusters of cardiometabolic conditions, such as obesity, diabetes, and chronic cardiac disease, are associated with both COVID-19 severity and cardiovascular complications90,91, raising the possibility of underlying mechanisms that are related to chronic low-grade inflammation92.
In addition to clusters of conditions revealing potential biological mechanisms, interactions between demographic and socioeconomic factors, ethnicity and environment also influence the likelihood of infection and subsequent outcomes. This highlights limitations to current approaches, which often isolate biomedical investigation from social context. A broader lens is needed to understand the full complexity and mechanisms that lead to poor outcomes in COVID-19 in relation to comorbidity and multimorbidity93. From a public-health perspective, the increasing prevalence of multimorbidity and the associated increased susceptibility to infectious disease, such as COVID-19, underscores the need to improve baseline health to mitigate the impact of future epidemics and pandemics. Although some risk factors, such as iatrogenic immunosuppression, are not directly modifiable, others such as obesity can be, and addressing these also requires efforts to reduce the socioeconomic inequalities that underpin them. The net effect of COVID-19, non-communicable diseases associated with adverse outcomes, and the socioeconomic basis of non-communicable disease prevalence can be considered a ‘syndemic’93,94.
From a health-services perspective, clinical management of patients with multimorbidity is often complex. Multimorbidity can increase the risk of harm arising through clinical interventions95. In addition, multimorbidity is associated with lower likelihood of survival, and a greater risk of impaired quality of life in those who do survive96. This means that clinical guidelines for COVID-19 management require nuanced interpretation and tailoring to individual circumstances. Furthermore, an individualized, careful balance of benefits and potential harms of treatments is needed, particularly when considering initiation of invasive, potentially burdensome treatments.
Future directions and conclusions
Identifying causal relationships between comorbidities and outcomes in COVID-19 is methodologically difficult but scientifically important. In particular, a deeper understanding of the causal relationships between clinically relevant traits and comorbidities and disease outcomes in COVID-19 may reveal new molecular mechanisms and opportunities for new or repurposed therapeutics. Host genetics techniques, including Mendelian randomization, will help eliminate confounding in these relationships. More intensive efforts are required to define the mechanistic relationships between comorbidities in epidemiological, host genetics, and laboratory research.
Recognition of the differences between the three phases of COVID-19 may enable future research to distinguish the specific impact of comorbidities on distinct mechanistic pathways. The impact of most common comorbid illnesses likely operates through a combination of defective resistance, defective tolerance, and the generic effect of frailty and reduced functional reserve. In this context, public-health efforts to improve baseline population health are an integral part of pandemic preparedness. In particular, the substantial burden of disease created by PASC, and the wider public-health implications of the ‘syndemic’ of COVID-19 on top of widespread comorbidity due to common non-communicable disease and socioeconomic inequality merit urgent attention.
Ongoing evolution of SARS-CoV-2, the potential for co-infections with other seasonal pathogens such as influenza, and altered baseline host resistance due to vaccination mean that patterns of disease will continue to change. The significant effect of comorbidity on disease outcome is likely to persist and should remain a research priority.