Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial
Abstract
Autism spectrum disorder (ASD) is defined by hallmark behaviors involving reduced communication and social interaction as well as repetitive activities and restricted interests. ASD represents a broad spectrum, from minimally affected individuals to those requiring intense support, with additional manifestations often including anxiety, irritability/aggression and altered sensory processing. Gastrointestinal (GI) issues are also common in ASD, and studies have identified changes in the gut microbiome of individuals with ASD compared to control populations, complementing recent findings of differences in gut-derived metabolites in feces and circulation. However, a role for the GI tract or microbiome in ASD remains controversial. Here we report that an oral GI-restricted adsorbent (AB-2004) that has affinity for small aromatic or phenolic molecules relieves anxiety-like behaviors that are driven by a gut microbial metabolite in mice. Accordingly, a pilot human study was designed and completed to evaluate the safety of AB-2004 in an open-label, single-cohort, multiple-ascending-dose clinical trial that enrolled 30 adolescents with ASD and GI symptoms in New Zealand and Australia. AB-2004 was shown to have good safety and tolerability across all dose levels, and no drug-related serious adverse events were identified. Significant reductions in specific urinary and plasma levels of gut bacterial metabolites were observed between baseline and end of AB-2004 treatment, demonstrating likely target engagement. Furthermore, we observed improvements in multiple exploratory behavioral endpoints, most significantly in post hoc analysis of anxiety and irritability, as well as GI health, after 8 weeks of treatment. These results from an open-label study (trial registration no. ACTRN12618001956291) suggest that targeting gut-derived metabolites with an oral adsorbent is a safe and well-tolerated approach to improving symptoms associated with ASD, thereby emboldening larger placebo-controlled trials.
Current estimates of ASD prevalence reach one in 54 children born in the United States1, and recent clinical failures2,3,4,5,6,7,8,9 highlight the need to expand drug development efforts. Behavioral features and severity are measured by validated observational assessment tools, as there are no imaging-based or molecular biomarkers that reliably and objectively diagnose ASD. Furthermore, autism manifests across a broad spectrum, from minimally affected individuals to those who require intense support, and there are no approved drugs for core symptoms. The etiology of ASD is poorly understood and likely multifactorial but is known to involve complex genetic risks10, with over 100 genes implicated to date, each with a small effect size11. A role for environmental risks in ASD has also been proposed, encompassing diet12, maternal infection13, exposure to toxins14 and changes in the gut microbiome15. The notion that fixed genetic predispositions coupled with variable environmental risks together manifest symptom severity is intriguing from a therapeutic perspective, because correcting mutations in the genome remains challenging, and reducing potential environmental contributors is likely more tractable. Recent studies suggest that molecules produced in the GI tract can enter systemic circulation and affect immunity16, metabolism17 and behavior18. Altered immune and metabolic profiles have been associated with various neuropsychiatric disorders, such as ASD19,20,21, and the microbiome and metabolome are altered in individuals with ASD19,22, anxiety23, depression24 and schizophrenia25. Notably, over a dozen studies have shown changes in the fecal microbiome in several ASD cohorts compared to controls26, although these associations do not resolve cause or effect. Dietary habits likely contribute to the ASD microbiome27. Several studies have also revealed changes in the metabolome of individuals with ASD from diverse geographies19,28,29,30, with reports showing dysregulation of the fecal metabolome19,31,32,33. We previously identified several gut microbial metabolites that correlate with ASD-like symptoms in mice, and administration of one of these metabolites, 4-ethylphenyl sulfate (4EPS), to naive animals induced an anxiety-like phenotype34. 4EPS and several structurally related molecules are dysregulated in the feces and plasma of individuals with ASD compared to control populations19. Furthermore, an open-label fecal transplant study described improvements in GI and behavioral parameters in 18 individuals with ASD35,36, with a partial restoration of metabolomic profiles37. Although these early studies remain speculative, the prospect for ASD interventions targeting the microbiome or metabolome are both conceptually attractive and practically realizable. AB-2004, known otherwise as AST-120 (ref. 38), is a high-surface-area spherical carbon adsorbent that has affinity for uremic toxins, including those of gut bacterial origin, such as the simple phenols, 4EPS, p-cresol sulfate (pCS) and p-cresol glucuronide (pCG), as well as the indole derivative 3-indoxyl sulfate (3IS) and hippuric acid39, based on evidence from rodent models and patients with chronic kidney disease40 and irritable bowel syndrome41. Taken orally, it binds and sequesters related aromatic metabolites as it passes through the GI tract without being absorbed and is ultimately excreted, effectively lowering systemic metabolite exposure. We hypothesized that AB-2004 would also reduce the structurally related 3-hydroxyhippurate (HHA) and phenylpropanoic acids or other related small-molecule metabolites, such as 3-(3-hydroxyphenyl)-3-hydroxypropionate (HPHPA), 3-(4-hydroxyphenyl)propionate (HPPA), 3-hydroxyphenylacetate (HPAA), 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF) and imidazolepropionate (IPA). There is accumulating evidence that increased levels of this chemical class of microbial metabolites is associated with ASD37,42,43. For instance, 4EPS, pCS, 3IS, hippuric acid and hydroxyphenylacetic acid metabolite levels have been found to be elevated in children with ASD19,20,28,42,44,45,46,47, and levels of some of these metabolites also correlate with GI and behavioral symptoms19,20,42,44. Unpublished preclinical work suggests that these findings might go beyond simple associations between microbial metabolites and behavioral endpoints; namely, production of 4EPS by gut bacteria results in changes in brain cell function and increases anxiety-like and ASD-like behaviors in mice (see accompanying manuscript, accepted for publication). pCS administered to mice leads to deficits in social communication and repetitive behaviors48, and both pCS and 3IS promote anxiety-like and depression-like features in rodents49,50,51Main
Results
AB-2004 reduces 4EPS and anxiety-like behavior in mice
We recently reported that 4EPS is elevated in the plasma of individuals with ASD34, although bacterial sources for production of the metabolite remained unknown. The gut microbiome is predicted to harbor genes that convert tyrosine, the precursor of several mammalian neurotransmitters, to 4-ethylphenol (4EP), which could then be sulfated to 4EPS. Sulfation in the liver or other organs is a common detoxifying activity in mice and humans for structurally related phenolic molecules52. We systematically tested several bacterial species for the enzymatic activity required for biosynthesis of 4EP from tyrosine and then cloned genes that showed predicted activity into genetically tractable strains of gut bacteria. Subsequently, gnotobiotic mice were colonized with isogenic bacterial strains that were engineered to convert tyrosine to 4-ethylphenol (4EP+ group) or mutants of the same strains that lack genes encoding enzymes that mediate this conversion (4EP− group). We verified that gut microbial production of 4EP, followed by efficient host sulfation, leads to the presence of 4EPS in urine of 4EP+ mice (Extended Data Fig. 1a–c; control). 4EPS promoted anxiety-like behavior in several testing paradigms: (1) open-field exploration where mice ventured less into the more exposed zone of the arena; (2) the elevated plus maze (EPM) where 4EP+ mice spent less time in the terminus of the open arms; and (3) the marble burying test (Extended Data Fig. 1f–i). (For further details, see Methods and the accompanying manuscript). This experimental paradigm enables effective testing of drugs that neutralize 4EPS.
After colonization, both groups of mice were placed on a diet consisting of 5% AB-2004 by weight of chow or matched control diet 2 weeks before behavior testing (Extended Data Fig. 1b). As expected, systemic levels of 4EPS measured in urine were lowered by oral AB-2004 administration (Extended Data Fig. 1c). There were no differences in bacterial colonization levels; weight gain was not significantly influenced (Extended Data Fig. 1d,e); and no signs of distress, illness or other differences were observed among the mouse groups. Behavioral analysis revealed that treatment with AB-2004 ameliorated behavioral deficits in the open field and EPM tests for anxiety (Extended Data Fig. 1f–h). Specifically, 4EP+ mice given AB-2004 spent more time exploring the exposed (that is, riskier) areas of the tests compared to 4EP+ mice on the control diet. AB-2004 treatment also stabilized performance in the anxiety/repetitive behavior-related marble burying test (Extended Data Fig. 1i). These results were accompanied by trending improvements in the grooming test for repetitive behavior (Extended Data Fig. 1j). Results from this simplified mouse model indicate that AB-2004 was effective in reducing systemic levels of 4EPS and preventing 4EPS-induced anxiety-like behaviors. These preclinical findings, coupled with the known safety profile of AB-2004, provided justification for a human trial.
Phase 1 clinical trial design
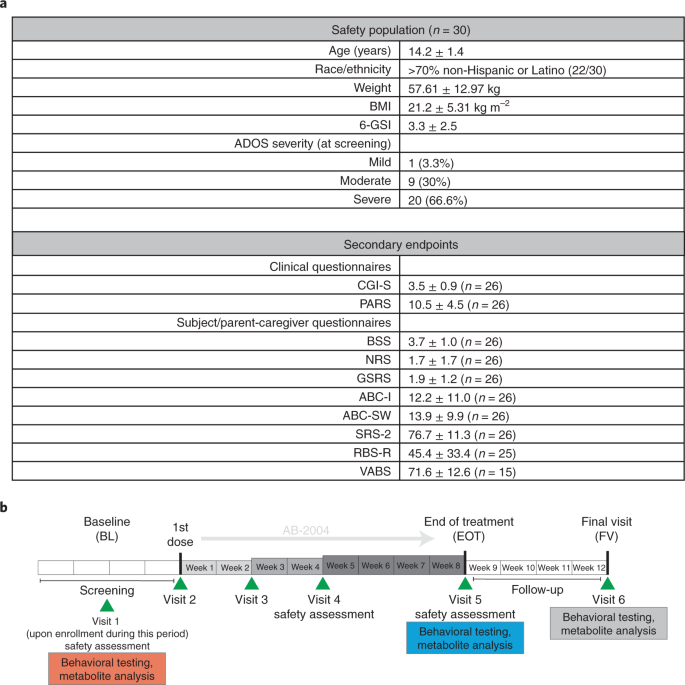
We designed and conducted an open-label, phase 1b/2a clinical trial at three sites in New Zealand and Australia with primary endpoints for safety and tolerability as determined by reported and observed adverse effects and laboratory results. Secondary endpoints included target engagement, which was assessed objectively by measuring microbially derived metabolites in plasma and urine. Behavioral endpoints were exploratory. At screening, ASD diagnosis was confirmed using the Autism Diagnostic Observational Schedule, 2nd Edition (ADOS-2), and the presence of GI symptoms was verified through the Gastrointestinal Severity Index (6-GSI)53 and a 14-d bowel habit diary. Thirty adolescents (29 males and one female) previously diagnosed with ASD (Fig. 1a and Extended Data Fig. 2a) met the inclusion criteria as outlined in Extended Data Table 1. After enrollment, each participant was administered baseline behavioral assessments using the Pediatric Anxiety Rating Scale (PARS)54, the Aberrant Behavior Checklist (ABC)55, the Social Responsiveness Scale (SRS-2)56, the Repetitive Behavior Scale Revised (RBS-R)57 and the Vineland Adaptive Behavior Score (VABS-3)58, and several gastrointestinal symptom metrics were measured, including the 6-GSI53, the Bristol Stool Scale (BSS)59 and the Gastrointestinal Symptom Rating Scale (GSRS)60. All inclusion and exclusion criteria can be found in Extended Data Table 1. Study participants were asked to take three daily, weight-adjusted (see Methods for details) ascending oral doses of AB-2004 totaling ≤2.25 g per day, escalating to ≤4.5 g per day at 2 weeks and ≤6 g per day daily at 4 weeks until the end of treatment on week 8, with a final visit 4 weeks after the end of treatment (Fig. 1b). Urine, blood and stool samples were collected, and behavioral assessment performed at baseline (BL), end of treatment (EOT) and final visit (FV), with continuous health monitoring throughout (Fig. 1b and Extended Data Table 2). Twenty-seven of 30 enrolled participants completed to the EOT, but one was removed due to Coronavirus Disease 2019 (COVID-19) schedule interruptions, resulting in a completers group of 26. Twenty-four participants completed to the FV.
a, Trial demographics and metadata summary of participants. b, Phase 1 clinical trial schedule. Participants were screened during a 4-week run-in period, followed by dose escalation in weeks 0–2, weeks 2–4 and weeks 4–8, with a follow-up 4 weeks after trial. ABC-SW, Aberrant Behavior Checklist-Social Withdrawal; BMI, body mass index.
AB-2004 is safe and well tolerated in adolescents with ASD
Assessment of overall health, including GI symptoms, was determined by the Clinical Global Impressions scale for severity and improvement (CGI-S and CGI-I, respectively). From BL to EOT, 76.9% of participants (20 of 26) improved at least one point on the CGI-I scale (Extended Data Fig. 2b). Although GI symptoms were an inclusion criterion based on 6-GSI and a 14-d bowel habit e-diary assessment during screening, 19.2% of participants presented with no clinical GI disorder based on the CGI at the time of assessment (including normal and borderline scores). Notably, the number of participants with no measurable GI symptoms doubled from BL to EOT (19.2% to 38.5%) (Extended Data Fig. 2c).
Median adherence to dosing was 97.5%, and no laboratory concerns arose, showing that AB-2004 was well tolerated. Notably, overall safety metrics showed that no serious adverse events related to the drug or any deaths occurred during the reporting period of the study. Most mild or moderate adverse effects were in the GI category, including abdominal pain and nausea (Table 1). The study, therefore, met its primary endpoints for safety and tolerability, extending the safety record of this drug to an adolescent ASD population for the first time.
Microbial metabolite levels are lowered by AB-2004
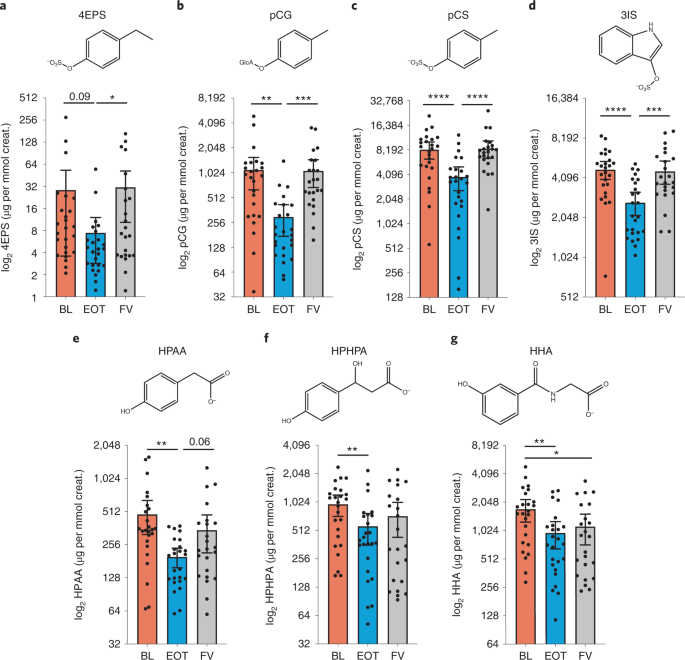
Based on the known pharmacology of binding phenolic compounds and its practically complete lack of systemic absorption, we hypothesized that oral AB-2004 would diminish levels of specific gut-derived metabolites in circulation by facilitating their excretion in the feces. As predicted, AB-2004 treatment resulted in reduced levels of 4EPS, pCG, pCS, 3IS, HPHPA and HPAA in urine from the BL to EOT time points (Fig. 2 and Extended Data Fig. 3a), with similar profiles in plasma (Extended Data Fig. 3b). Concentrations between urine and plasma were highly correlated for many metabolites (Extended Data Fig. 3c). Urine metabolites largely rebounded to pre-drug (BL) levels at the FV time point 4 weeks after treatment had concluded, supporting the conclusion that metabolite levels were influenced directly by AB-2004 administration (Fig. 2). N-acetyl serine levels, which were measured as a control metabolite not bound by AB-2004, did not change in urine or plasma between BL and EOT (Extended Data Fig. 3d). These data indicate that target engagement of gut-derived microbial metabolites by AB-2004 can effectively reduce their systemic levels.
a–f, Metabolite levels in urine from BL (orange), EOT (blue) and FV (gray) time points from all participants, normalized to creatinine (µg metabolite per mmol creatinine) on log2 scale. Exact adjusted P values: 4EPS, 0.03; pCG, 0.002, 0.0003; pCS, <0.0001, <0.0001; 3IS, <0.0001, 0.0003; HPAA, 0.002; HPHPA, 0.0095; and HHA, 0.005, 0.02. Chemical structures are shown above associated data panels. Urine from one participant could not be obtained due to incontinence. Data analysis was conducted on the completers group (n = 26, with two missing data points in the FV time point because two participants missed assessment due to parental illness). Analysis is exploratory and post hoc in nature, shown as mean and 95% confidence interval analyzed with a linear mixed-effects model with Geisser–Greenhouse correction, multiple comparisons and false discovery rate correction across all metabolites (nominal P values: *Padj ≤ 0.05; **Padj ≤ 0.01; ***Padj ≤ 0.001; ****Padj ≤ 0.0001). creat., creatinine.
Oral AB-2004 might alter brain connectivity
As a preliminary look into potential brain activity patterns, we performed resting state functional magnetic resonance imaging (fMRI) on a small subset of ten study participants to estimate connectivity between brain regions. Two 5-min scans were conducted at BL and EOT time points focusing on changes in regions associated with emotional behavior responses. This included regions such as the amygdala, which is crucial for emotional processing networks such as those involving anxiety61, and the anterior cingulate cortex (ACC), which is involved in emotional and cognitive networks62. Atypical activity in one or both of these regions has been observed in preclinical studies63 and in ASD cohorts64,65,66,67,68. We observed a decrease in coupling between the amygdala and the rostral anterior cingulate cortex (rACC) (Extended Data Fig. 4a), an encouraging outcome because higher amygdala-rACC connectivity is associated with higher anxiety69. This pilot finding suggests that further study of functional connectivity in the amygdala and ACC might provide insights into mechanisms of action of AB-2004 and the metabolites it binds.
Exploratory outcomes show improvement of core ASD behavior
In addition, we captured several domains of behavioral data for all study participants as exploratory endpoints. The VABS58 was administered at BL, EOT and FV, and overall scores, as well as communication and socialization scores, were significantly increased by EOT (Extended Data Fig. 4b–e). Although ten participants were removed from this analysis owing to incomplete data, thereby limiting the sample size of this comparison, a mean increase of 7.8 points from BL to EOT in the Adaptive Behavior Composite score is above the minimal clinically relevant cutoff of 3.75 points70,71 (Extended Data Fig. 4b). Modest indications of improved social communication and repetitive behavior of study participants was also detected in other behavioral assessments, including the SRS (Extended Data Fig. 5a–f) and, to a lesser extent, in the ABC (Extended Data Fig. 5g–j). Importantly, for our study, both assessment tools have been used to evaluate the efficacy of ASD therapeutics72,73,74. Future studies will aim to confirm the encouraging, but preliminary, improvements observed here in social communication and repetitive behavior, as these outcome measures are likely to be influenced by placebo effects75.
AB-2004 reduced anxiety and irritability
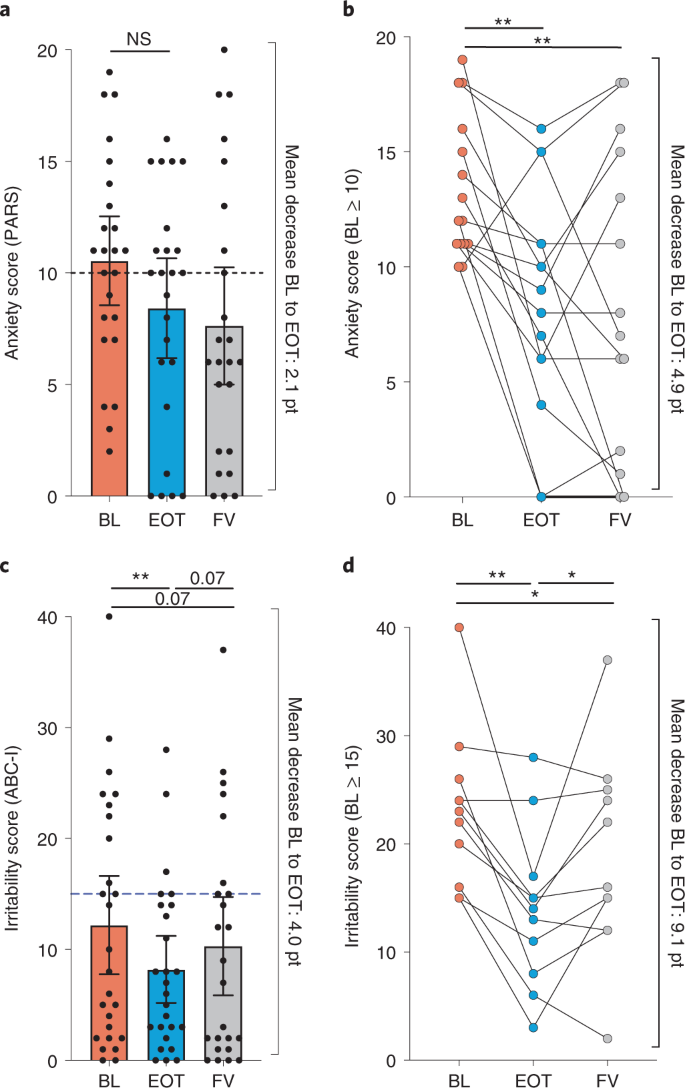
We report the most striking behavioral outcomes of AB-2004 treatment in two highly prevalent non-core domains of ASD, namely anxiety and irritability. In particular, study participants with elevated BL anxiety scores ≥10, as measured by the PARS test, showed marked improvements in anxiety between initial (BL) and last (EOT) dose, a positive effect that persisted 1 month after withdrawal of drug (FV) (Fig. 3a,b). These results indicate that AB-2004 might be effective in treating elevated anxiety in individuals with ASD, as the minimum clinically important change in the PARS score is 15%76, and our results show an average improvement of 30% in the group with anxiety at BL, with nine of 15 individuals with elevated anxiety, resulting in a diagnostic score that qualified as remission (score of 10 or lower).
a, Anxiety (measured by PARS) scores of all eligible study participants at BL (orange), EOT (blue) and FV (gray) time points, with mean test scores represented as bars. Dotted line indicates threshold for anxiety (n = 24). b, PARS anxiety scores of the subset of participants scoring ≥10 at BL, showing participant change to EOT and FV (n = 15). Exact adjusted P values: BL versus EOT, 0.001; BL versus FV, 0.001. c, Irritability (measured by ABC-I) scores of all eligible study participants at BL, EOT and FV time points. Dotted line indicates threshold for the top quartile of irritability severity among the ASD population (BL and EOT, n = 26; FV, n = 24 owing to a missing FV value for two participants). Exact adjusted P value: 0.008. d, Irritability (ABC-I) scores of the subset of participants scoring ≥15 at BL, showing participant change to EOT and FV (n = 11). Exact adjusted P values: BL versus EOT, 0.003; EOT versus FV, 0.04; BL versus FV, 0.04. Data analysis was conducted on the completers group (n = 26, with two missing data points at the FV time point in ABC-I and at all time points in PARS because two participants missed assessments due to parental illness). Analysis is exploratory and post hoc in nature, shown as mean and 95% confidence interval. Analyses were performed by repeated-measures one-way ANOVA (a, b and d) or the linear mixed-effects model (c) with Geisser–Greenhouse correction, multiple comparisons and false discovery rate correction within each test (nominal P values: *Padj ≤ 0.05; **Padj ≤ 0.01). NS, not significant.; pt, points.
Irritability is also frequent in the ASD population and can be assessed as part of the ABC scale55. We observed a significant overall decrease in irritability as measured by the ABC-I subscale between BL and EOT (Fig. 3c). In particular, participants with high BL irritability (scores ≥15), which represents the top quartile of severity within the ASD population as a whole77, displayed a remarkable 9.1-point decrease in ABC-I (Fig. 3d). The improvements at EOT were largely mitigated after drug washout (FV) in most participants (Fig. 3d). Correlations between a single metabolite and behavioral scores were not clear (Extended Data Table 3), and analysis to understand the potential effect of multiple metabolite interactions is underway. These data reveal that almost all study participants with elevated anxiety or irritability showed significant behavioral improvements after 8 weeks of treatment with AB-2004.
Discussion
Based on data from the recently completed open-label trial described here, AB-2004 is safe and well tolerated for use in an adolescent ASD population, with no serious adverse events related to the drug. Our studies also suggest target engagement by AB-2004, as evidenced by reduced levels of gut microbial metabolites in plasma and urine after 8 weeks of treatment and a general rebound to BL levels after 4 weeks of drug washout. Furthermore, AB-2004 decreased the number of participants presenting with GI symptoms; however, it is unclear whether intestinal issues are linked to other endpoints. Although our study was powered for safety and tolerability, we surprisingly observed indicators of improvements in ASD-associated behaviors, namely anxiety and irritability. Decreased anxiety persisted after drug removal, whereas improvements in irritability largely returned to BL levels by the FV. We cannot exclude a contribution for metabolites bound by AB-2004 that were not measured here, of host, dietary or microbial origin. Also, our study does not resolve indirect effects of drug through potential changes in nutrition, immune status and GI function, for example, and further proof of mechanism will require additional work. However, to our knowledge, this is the first interventional study linking phenolic metabolites in the gut with clinical features of ASD. Although the preliminary evidence for improvements in behavior within this small ASD cohort are encouraging, the absence of a control arm necessitates double-blinded, placebo-controlled trials to confirm the efficacy of AB-2004.
There are currently no approved pharmacological therapies for the treatment of the core symptoms of ASD78. Two drugs, risperidone and aripiprazole, are currently approved by the US Food and Drug Administration (FDA) for treatment of irritability in individuals with ASD79. Irritability behaviors are common in pediatric ASD and have major implications in child development, receptivity to behavioral therapy and child/caregiver health-related quality of life80. Both drugs are atypical anti-psychotic medications and are associated with a range of side effects7,81, such as somnolence, metabolic changes, weight gain, leukopenia and tardive dyskinesia73,82,83,84. In a phase 3 study of aripiprazole85 with inclusion criteria based on high irritability levels (ABC-I ≥18), the response rate, or percentage of individuals with 25% improvement in ABC-I scores and a CGI-I ≤2, was 49–56% in the drug arms, with a 34.7% response rate in the placebo arm. In our study, a post hoc analysis showed a 75% and 82% response rate in subgroups meeting somewhat similar criteria (ABC-I 18 or 15, respectively), providing optimism for future, larger placebo-controlled studies with AB-2004. Importantly, ABC-I is a validated instrument for measuring irritability in individuals with ASD and was used as the primary efficacy measurement in pivotal trials leading to FDA approval of aripiprazole and risperidone; therefore, a regulatory path for the development of AB-2004 in this patient population is already established. The favorable clinical safety profile makes AB-2004 an attractive potential alternative to the currently available drugs for irritability in ASD. Based on findings from this safety and tolerability study, a placebo-controlled randomized trial is now underway to test the effects of AB-2004 in an ASD cohort powered to report changes in irritability.
Methods
Preclinical methods
Mouse husbandry
All animal husbandry and experiments were approved by the California Institute of Technology Institutional Animal Care and Use Committee. Throughout the study, colonized animals were maintained in autoclaved microisolator cages with autoclaved bedding (Aspen Chip Bedding, Northeastern Products), water and chow. Standard chow was provided to the animals (Laboratory Autoclavable Rodent Diet 5010, LabDiet) until diet switch to irradiated 5% AB-2004 or control diets (Teklad) at 5 weeks. This percentage of AB-2004 (AST-120) in mouse chow has been previously used safely in mice34,86,87. Mice were maintained at an ambient temperature of 71–75 °F, 30–70% humidity, at a cycle of 13 h light and 11 h dark.
Experimental design of mouse experiments
Germ-free C57BL/6J male mouse (Mus musculus) weanlings (3 weeks of age) from the Mazmanian laboratory colony were colonized by gavage of 100 µl of a 1:1 mixture of 109 colony-forming units per milliliter of Bacteroides ovatus (± 4EP pathway genes) and wild-type Lactiplantibacillus plantarum. Urine was collected at 7 weeks before behavior testing. Behavior testing began at 7 weeks of age, 3 d after urine collection.
Analysis of metabolites from urine of mice
Urine was passively collected, and 4EPS levels were quantified by liquid chromatography–mass spectrometry (LC–MS) and normalized to creatinine levels by Charles River Laboratories.
Behavior testing
Behavior testing was performed as previously described34,86,87. All mice were tested using the same battery of behavioral tests, starting at 6 weeks of age, in the following order: EPM, open-field testing, marble burying and grooming. Mice were allowed to settle for at least 2 d after cage changing before they were tested, and tests were performed 2–3 d apart to allow mice to rest between tests. Mice were acclimated to the behavior testing room for 1 h before testing. Mice were tested during the light phase of the light/dark cycle.
Clinical methods
Clinical study design and ethical approval
The AXL-2004-001 study (trial registration no. ACTRN12618001956291) (ANZCTR (88,89)) was an open-label, outpatient, multiple-ascending-dose phase 1b/2a study in an ASD-diagnosed adolescent (12–17 years old) population with confirmed GI symptoms (for example, diarrhea, constipation, abdominal pain and bloating). Forty-one individuals were screened between 18 April 2019 and 23 January 2020. Thirty participants were enrolled across three sites in Australia and New Zealand, including the Queensland Children’s Hospital in Brisbane (14 participants), the Brain and Mind Centre in Sydney (six participants) and Optimal Clinical Trials in Auckland (ten participants). There was no formal sample size calculation for this phase 1 study because it focused on safety and tolerability. This approach is common in early-stage exploratory clinical trials. All necessary licenses and permissions to use the behavioral assessments outlined in the study protocol were obtained before initiating the study.
The study protocol, investigator brochure, participant information and consent forms, participant-facing questionnaires, recruitment documentation and procedures and documentation regarding the investigatorsʼ experience and qualifications were submitted to Health and Disability Ethics Committees (New Zealand), Children’s Health Queensland Hospital and the Health Service Human Research Ethics Committee and Bellberry Human Research Ethics Committee for ethical review and approval. The study was conducted in accordance with the Declaration of Helsinki (Fortaleza, October 2013), ICH E6 guidelines, Good Clinical Practice and local regulations.
Study participation
This open-label study consisted of four different dosing plans based on participant weight at visit 1. Eligible participants were escalated through three dosing periods during the 8-week treatment period, starting with the lowest dose for their dosing plan (see Supplementary Methods for more details). Participants were requested to consume AB-2004 90 min after any other concomitant medications. Safety and tolerability were confirmed before a participant escalated to the next dosing level. If participants were unable to tolerate a dosing level, they were returned to the previous dosing level for the remainder of the treatment period. After the last dose of AB-2004, participants returned to the clinic 28 d later for a follow-up safety evaluation (FV). The last visit of the study was completed on 15 May 2020. Patient data were collected using IMednet (version 1.94.0). An e-diary by Dedo (www.dedo.ai) was designed to collect GI data.
Study participants and study populations
A total of 41 adolescent individuals, aged 12–17 years inclusive, were screened for eligibility for participation in the study, and the 30 who met the study-specific eligibility criteria were enrolled and received at least one dose of AB-2004 (Safety Population). Of the 41 individuals screened and 30 enrolled, 40 and 29, respectively, were male. A predominantly male cohort was targeted to reduce variability in response in this exploratory study that surveyed a wide range of behavioral assessments. One participant withdrew after the first dose due to the investigator’s decision based on the participant presenting with an unrelated viral infection. Another participant withdrew consent during the low-dose period due to anticipated admission to hospital for pre-existing behavioral difficulties. One participant withdrew due to significant study non-compliance, and two did not complete FV assessments due to the caregivers being unwell and unable to accompany the participants. A total of 27 participants (26 males and one female) completed at least up to the EOT visit (Completers Population). One participant, the female participant, was included in the Safety Population but was not included in the exploratory efficacy analysis. This participant was removed from the exploratory efficacy analysis because their participation in the trial coincided with the initial COVID-19 pandemic outbreak and its associated societal restrictions put into effect in Australia. These restrictions prevented the participant from conducting normal routines and accessing normal services. As determined by the site principal investigator, these abrupt changes in routine had an effect on the behavior of the participant; therefore, this participant was excluded from the efficacy analysis.
Safety assessments
The primary endpoint of the study was the safety and tolerability of AB-2004 as assessed by physical examinations, vital signs, clinical laboratory measurements (hematology, serum chemistry and urinalysis) and adverse events.
Blood collection
Blood was obtained using uniform collection kits from Sonic Clinical Trials sent to each facility. Blood was drawn from study participants on visits 1, 4 and 5 and aliquoted for health monitoring by Sonic Clinical Trials and metabolite analysis by Metabolon. Blood chemistry panels performed by Sonic Clinical Trials included albumin, alkaline phosphatase, alanine amino transferase, aspartate amino transferase, blood urea nitrogen, urea, corrected calcium, bicarbonate, chloride, creatinine, gamma-glutamyl transpeptidase, glucose, lactate dehydrogenase, magnesium, phosphorus, potassium, sodium, total bilirubin, conjugated bilirubin, unconjugated bilirubin and total protein. Hematology panels included measurement of platelets, hematocrit, red blood cells, hemoglobin, reticulocytes, total white blood cell count and absolute and percentages of neutrophils, lymphocytes, monocytes, eosinophils and basophils.
Urine collection
Participants were provided with a urine home collection kit and instructions to collect all of the first morning void a maximum of 2 d before clinic visit and place in a refrigerator to bring to their visit or to be picked up by courier. Urinalysis samples were collected during the in-clinic visit. Aliquoting for metabolite analysis and health monitoring urinalysis was performed by Sonic Clinical Trials and included measurements of pH, specific gravity, ketones, protein, glucose, nitrite, urobilinogen, leukocyte esterase and blood.
Human plasma metabolite quantification
Human plasma was analyzed by Metabolon. In brief, plasma was spiked with internal standards (4-ethylphenyl sulfate-d4, p-cresol sulfate-d7, 3-hydroxyhippurate-13C2,15N, 3-hydroxyphenylacetate-d3, 3-(3-hydroxyphenyl)-3-hydroxypropionate-d3, 3-indoxyl sulfate-13C6, 3-(4-hydroxyphenyl)propionate-d4, p-cresol glucuronide-d7 and N-acetylserine-d3,), protein precipitated and analyzed on an Agilent 1290/AB Sciex 5500 QTrap LC–MS/MS system equipped with a UHPLC C18 column. Quantitation was performed using a weighted linear least squares regression analysis with a weighting of 1/x or 1/x2 generated from fortified calibration standards prepared immediately before each run.
Human urine metabolite quantification
Human urine was analyzed by Metabolon. In brief, urine was diluted ten-fold and spiked with internal standards (p-cresol sulfate-d7, 3-hydroxyhippurate-13C2,15N, 3-hydroxyphenylacetate-d3, 3-(3-hydroxyphenyl)-3-hydroxypropionate-d3, 3-indoxyl sulfate-13C6, 3-(4-hydroxyphenyl)propionate-d4, p-cresol glucuronide-d7 and N-acetylserine-d3,), and then an aliquot was subjected to either a solvent crash (for p-cresol sulfate, 3-indoxyl sulfate and p-cresol glucuronide) or derivatization (for 3-hydroxyhippurate, 3-hydroxyphenylacetate, 3-(3-hydroxyphenyl)-3-hydroxypropionate, N-acetylserine and 3-(4-hydroxyphenyl)propionate) and analyzed on an Agilent 1290/AB Sciex 5500 QTrap LC–MS/MS system equipped with a UHPLC C18 column in negative mode. Quantification of 4EPS was performed by the same method with a solvent crash (using the internal standard, 4-ethylphenyl sulfate-d4) but without sample dilution. Quantitation was performed using a weighted linear least squares regression analysis with a weighting of 1/x generated from fortified calibration standards prepared immediately before each run. All urine metabolites were normalized to creatinine levels.
Exploratory efficacy assessments
Exploratory efficacy outcomes included changes from BL at EOT and FV on the GSI-6, Numerical Rating Scale (NRS), GSRS, BSS, RBS-R VABS, CASI-5, SRS, CGI-S and CGI-I, ABC or PARS diagnostics. Efficacy assessments were administered on site at the respective clinics during visits. VABS, PARS and CGI-S and CGI-I were conducted by the principal investigator or qualified designee. The GSI-6, NRS, GSRS, RBS-R, BSS, CASI-5 SRS and ABC questionnaires were completed by the designated caregivers of the participants. In the VABS assessment, ten participants did not pass the under 25% estimated answers criterion of any domain during assessment and, thus, had to be removed from this analysis, according to the VABS manual, page 47 (ref. 5).
AB-2004 treatment dosage
For individuals weighing ≥60 kg, three daily doses each of:
Period 1: 0.75 g, Days 1–14 (2 weeks)
Period 2: 1.5 g, Days 15–28 (2 weeks)
Period 3: 2 g, Days 29–56 (4 weeks)
For individuals weighing 50–59 kg, three daily doses each of:
Period 1: 0.75 g, Days 1–14 (2 weeks)
Period 2: 1.0 g, Days 15–28 (2 weeks)
Period 3: 1.75 g, Days 29–56 (4 weeks)
For individuals weighing 40–49 kg, three daily doses each of:
Period 1: 0.5 g, Days 1–14 (2 weeks)
Period 2: 0.75 g, Days 15–28 (2 weeks)
Period 3: 1.5 g, Days 29–56 (4 weeks)
For individuals weighing 30–39 kg, three daily doses each of:
Period 1: 0.5 g, Days 1–14 (2 weeks)
Period 2: 0.75 g, Days 15–28 (2 weeks)
Period 3: 1.0 g, Days 29–56 (4 weeks)
Magnetic resonance
Scan parameters
All scans were collected using phased array receive-only head coils (32 channels at sites 1 and 2, 64 channels at site 3).
High-resolution anatomic images (T1-weighted (T1w) and T2-weighted (T2w)) were acquired with 1-mm isotropic resolution. T1w images (2@4:01 each, for sites 1 and 2, and 1@4:00 for site 3) were sagittally oriented using a 3D MPRAGE sequence. A single resolution matched T2w image (4:28) was acquired (the T2_CUBE sequence at site 1, T2_SPACE at sites 2 and 3).
Two gradient echo multi-band echo planar imaging (EPI) rs-FMRI acquisitions (300 volumes each) were performed with 2.5-mm isotropic resolution, 1-s repetition time and multi-band factor 3. In total, 51 slices were acquired obliquely, with the bottom slice oriented on the line between the bottom of the cerebellum and the bottom of the orbitofrontal cortex. The phase encode was reversed between the first and second scan (AP for the first scan, PA for the second) to allow for distortion correction.
Two diffusion scans were also acquired as part of the protocol (5:56 each), but they were not used for this analysis.
Data processing
Before processing, all data were named and organized following the BIDS 1.2.1 specification. Anatomical and fMRI data used in this manuscript were pre-processed using fMRIPrep 20.0.4 (ref. 90) (RRID: SCR_016216), which is based on Nipype 1.4.2 (ref. 91) (RRID: SCR_002502).
Anatomical data pre-processing
A total of two T1w images were corrected for intensity non-uniformity (INU) with N4BiasFieldCorrection92, distributed with ANTs 2.2.0 (ref. 93) (RRID: SCR_004757) per individual. The T1w reference was then skull-stripped with a Nipype implementation of the antsBrainExtraction.shworkflow (from ANTs), using OASIS30ANTs as target template. Brain tissue segmentation of cerebrospinal fluid (CSF), white matter (WM) and gray matter (GM) was performed on the brain-extracted T1w using fast (FSL 5.0.9, RRID: SCR_002823)94. A T1w reference map was computed after registration of four T1w images (after INU correction) using mri_robust_template (FreeSurfer 6.0.1)95. Brain surfaces were reconstructed using recon-all (FreeSurfer 6.0.1, RRID: SCR_001847)96, and the brain mask estimated previously was refined with a custom variation of the method to reconcile ANTs-derived and FreeSurfer-derived segmentations of the cortical GM of Mindboggle (RRID: SCR_002438)97. Volume-based spatial normalization to two standard spaces (MNI152NLin6Asym and MNI152NLin2009cAsym) was performed through non-linear registration with antsRegistration (ANTs 2.2.0), using brain-extracted versions of both T1w reference and the T1w template. The following templates were selected for spatial normalization: FSL’s MNI ICBM 152 non-linear 6th Generation Asymmetric Average Brain Stereotaxic Registration Model98 (RRID: SCR_002823; TemplateFlow ID: MNI152NLin6Asym) and ICBM 152 Nonlinear Asymmetrical template version 2009c98,99 (RRID:SCR_008796; TemplateFlow ID: MNI152NLin2009cAsym).
Functional data pre-processing
For each of the four blood oxygenation level dependent (BOLD) runs found per participant (across all tasks and sessions), a reference volume and its skull-stripped version were generated using fMRIPrep. A deformation field to correct for susceptibility distortions was estimated based on fMRIPrep’s fieldmap-less approach. Registration is performed with antsRegistration (ANTs 2.2.0), and the process is regularized by constraining deformation to be non-zero only along the phase-encoding direction and modulated with an average fieldmap template100. A corrected EPI reference was calculated. The BOLD reference was then co-registered to the T1w reference using bbregister (FreeSurfer)101. Co-registration was configured with 6 degrees of freedom. Head motion parameters with respect to the BOLD reference were estimated before any spatiotemporal filtering using mcflirt (FSL 5.0.9)102. BOLD runs were slice time corrected using 3dTshift from AFNI 20160207 (ref. 103) (RRID: SCR_005927). The BOLD time series were resampled onto their original, native space by applying a single composite transform to correct for head motion and susceptibility distortions. The BOLD time series were resampled into standard space, generating a pre-processed BOLD run in MNI152NLin6Asym space. First, a reference volume and its skull-stripped version were generated using a custom methodology of fMRIPrep. Automatic removal of motion artifacts using independent component analysis (ICA-AROMA)104 was performed on the pre-processed BOLD on MNI space time series after removal of non-steady-state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6 mm full width at half maximum. Corresponding ‘non-aggressively’ de-noised runs were produced after such smoothing. Additionally, the ‘aggressive’ noise regressors were collected and placed in the corresponding confounds file. Several confounding time series were calculated based on the pre-processed BOLD: framewise displacement (FD), DVARS and three region-wise global signals. FD and DVARS were calculated for each functional run, both using their implementations in Nipype (following the definitions by Power et al.105). The three global signals were extracted within the CSF, the WM and the whole brain masks. Additionally, a set of physiological regressors was extracted to allow for component-based noise correction (CompCor)106. Principal components were estimated after high-pass filtering the pre-processed BOLD time series (using a discrete cosine filter with 128-s cutoff) for the two CompCor variants: temporal (tCompCor) and anatomical (aCompCor). tCompCor components were then calculated from the top 5% variable voxels within a mask covering the subcortical regions. This subcortical mask was obtained by heavily eroding the brain mask. For aCompCor, components were calculated within the intersection of the mask and the union of CSF and WM masks calculated in T1w space, after their projection to the native space of each functional run (using the inverse BOLD-to-T1w transformation). Components were also calculated separately within the WM and CSF masks. For each CompCor decomposition, the k components with the largest singular values were retained, such that the retained components’ time series were sufficient to explain 50% of variance across the nuisance mask (CSF, WM, combined or temporal). The head motion estimates calculated in the correction step were also placed within the corresponding confounds file. The confound time series derived from head motion estimates and global signals were expanded with the inclusion of temporal derivatives and quadratic terms for each107. Frames that exceeded a threshold of 0.5 mm FD or 1.5 standardized DVARS were annotated as motion outliers. Gridded (volumetric) resamplings were performed using antsApplyTransforms (ANTs) and configured with Lanczos interpolation to minimize the smoothing effects of other kernels107. Non-gridded (surface) resamplings were performed using mri_vol2surf (FreeSurfer).
fMRI data analysis
To quantify connectivity between the bilateral amygdala and rACC, a region of interest (ROI) approach was used employing methods from our previous work108. The bilateral amygdala was defined using the Harvard-Oxford atlas. The rACC ROI is just anterior to the genu of the corpus callosum and was used in our previous work108,109. Average time courses for each ROI were extracted, demeaned, detrended, Hamming windowed and correlated to generate a single correlation value (r) for each participant both before and after treatment.
Statistical information
Results presented here are from post hoc analyses of the data from the clinical trial using GraphPad Prism 9. Here we present bar graphs representing the preclinical data by mean ± s.e.m. analyzed by ordinary two-way ANOVA test with false discovery rate correction using the Benjamini, Krieger and Yekutieli method, with individual variances computed for each comparison. Clinical data are presented as mean, and 95% confidence intervals were analyzed by repeated-measures ANOVA or linear mixed-effects model, with Geisser–Greenhouse correction tests and false discovery rate correction by the Benjamini, Krieger and Yekutieli method. Metabolite data are presented as individual graphs but were statistically analyzed across all metabolites and samples. Clinical behavioral metrics were analyzed within each test. Two-tailed Pearson’s correlations were performed comparing change in metabolite levels to change in behavioral scores for the PARS and ABC-I tests. fMRI values were analyzed using a two-tailed paired t-test. Study participants were studied as a single group, and all comparisons, especially those within the subgroup of participants in the top quartile of ASD severity, were post hoc and exploratory in nature. Missing data were not imputed, and data were analyzed for individuals who withdrew from the study, for any reason before study completion, regardless of treatment duration, up to the point of discontinuation.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data generated or analyzed during this study are included in this published article, and individual de-identified participant data can be found in the Supplementary Information. Axial Therapeutics (‘Axial’), under certain restrictions below, will provide access to at least the minimum datasets from its clinical trials that are necessary to interpret, verify and extend the research findings. Axial considers that the minimum datasets might include the study protocol (already provided as Supplementary Data) and the tables, lists and figures from the final Clinical Study Report. Such datasets will be made available only to qualified scientific researchers for legitimate scientific research purposes. Simultaneously, Axial is mandated by law and otherwise is committed to protecting the privacy and rights of all individuals who participate in its clinical trials; therefore, all data supplied will be de-identified according to applicable regulations. Data requests should be sent to the corresponding author, A. Stewart Campbell, at [email protected] (subject line: ‘AB-2004 Clinical Trial Data Request’). All data requests must be clearly described in a written proposal, including statement of research purpose, research plan and methods (including statistical analysis plan, if applicable), key research personnel and data sharing plans. All requests meeting submission requirements will be promptly considered for scientific merit by internal and external (if necessary) subject matter experts and compliance personnel, and a written response approving or denying access to the data will be provided. Approved requests will result in access to the necessary data through a secure data sharing portal, and access will be granted for a pre-specified defined period of time that is commensurate with the research plan. Under approved requests, Axial and the interested party must enter a data sharing agreement that will govern the terms and conditions for use, storage and communication of the data and terms for co-authorship resulting from any publication of the research results. It is recognized that certain regulations might apply in different countries, states or regions that might affect what data may be shared and with whom; Axial cannot guarantee access to the requested data in these circumstances. Source data are provided with this paper.