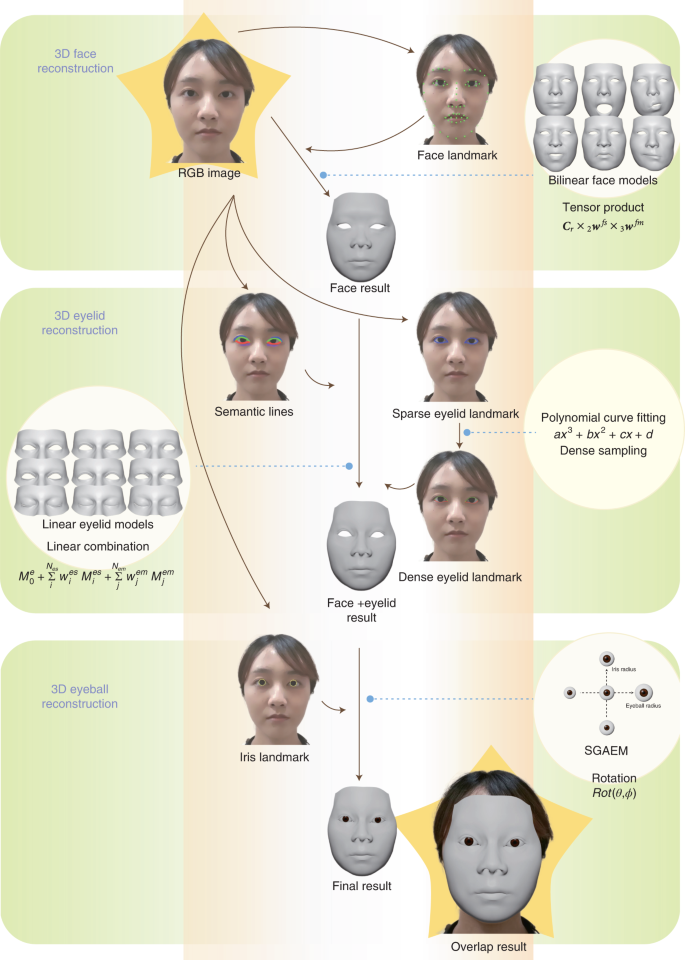
The workflow of the DM
In this work, the proposed DM patient privacy protection technology was based on the complementary use of deep learning and 3D reconstruction. Deep learning achieved feature extraction from different facial parts, and 3D reconstruction automatically digitalized the shapes and motions of 3D faces, eyelids and eyeballs based on the extracted facial features (Fig. 1). Different from other face reconstruction methods10,11,12,13,14,15,16, the proposed technology focused on accurate ocular reconstruction, including both shapes and movements.
In 3D reconstruction, we used three predefined parametric models for faces, eyelids and
eyeballs. The face model was mathematically a bilinear model17 (Methods) that represented a 3D face as a shape vector wfs and a motion vector wfm. Given a particular wfs and a wfm, the bilinear face model can reconstruct a particular 3D face mesh Mf. The face model can represent the overall geometry of the face, but eye regions lack details. Since the eye regions are important for diagnosis, we used a linear eyelid model18 to represent detailed eye regions. Similar to the face model, given this eyelid model, a detailed eyelid Me (of one eye) was represented by an eye shape vector wes and an eye motion vector wem. To additionally reconstruct the eyeballs, we used the simplified geometry and appearance eyeball model (SGAEM), introduced in our previous study19. The model approximated eyeballs as spheres and used three parameters, the eyeball radius re, the iris radius ri and the position pe relative to the face, to represent the static properties of an eyeball and the eyeball rotation in polar coordinates to represent eyeball motion.
Deep-learning techniques were leveraged to extract facial features that were used to infer the aforementioned model parameters to obtain the facial reconstruction results. First, a pretrained neural network was used as a face landmark detector to extract two-dimensional (2D) face landmarks Lface from an input red, green and blue color space (RGB) image. With the landmarks Lface, we estimated the face pose T (rotation and translation), face shape vector wfs and face motion vector wfm by minimizing the Euclidean distance between the 2D landmarks Lface and the 2D projections of the corresponding points on the 3D face Mf. Second, an eyelid landmark detector was used to extract 2D eyelid landmarks Leyelid and an eyelid semantic line detector was used to extract 2D eyelid semantic lines Seyelid. These two detectors were also neural networks trained by deep-learning techniques. Then, we similarly estimated the eyelid shape vector wes and the eyelid motion vector wem by minimizing the Euclidean distance between the 2D landmarks Leyelid and the projections of the corresponding points on the eyelid mesh Me, as well as by making the projected points on the semantic lines close to the detected semantic lines Seyelid on the image. Here, semantic lines provided rich and continuous information on the eyelid area, while landmarks were robust discrete features for tracking eyelid motions. Combining these two types of features made the reconstruction more accurate and stable. Finally, for the eyeballs, we trained another neural network as an iris landmark detector to extract 2D iris landmarks Liris from the input RGB image. As the eyeball radius re, iris radius ri and relative position pe were invariant in a video, we predicted them in the first frame and then fixed them in the following video frames. Per-frame eyeball rotations were estimated by minimizing the Euclidean distance between the 2D landmarks Liris and the projections of the corresponding points on the SGAEM. The DM included optional operations for adapting to different clinical applications, such as dealing with eye occlusion in videos recording the alternate cover test or reconstructing eyebrow movements in diagnosing ocular diseases.
Quantitative evaluation of the DM
The feasibility of the proposed model was evaluated on a video dataset of patients in the clinical trial. From May 2020 to September 2021, 405 participants, 187 (46.2%) males, aged 4 months to 61 years, who agreed to participate in the prospective study at the Digital Mask Program either by themselves or via their legal guidance; the participants consisted of (1) 100 outpatients from strabismus departments; (2) 92 outpatients from pediatric ophthalmology departments; (3) 102 outpatients from TAO departments; and (4) 111 outpatients from oculoplastic departments (Extended Data Table 1). In total, 253 (62.47%) of the 420 patients were diagnosed with ocular diseases on the basis of face-to-face assessments of the patients’ eyes.
To evaluate the applicability of the model, different cameras, including a Nikon 3500, Huawei p30 and Sony 4k, were used for video collection according to the following standards. The whole appearances of participants were collected from a distance ranging from 33 cm to 1 m according to the specific ocular examination. These videos were taken under room illuminance ranging from 300 to 500 lx.
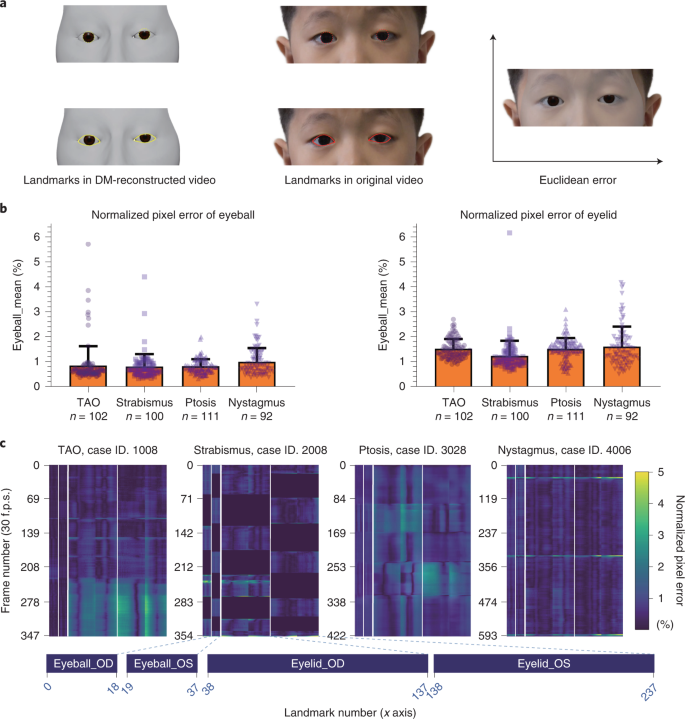
We used the proposed DM to process all the videos and quantitatively evaluated the reconstruction performance of the DM. In the quantitative evaluation, the performance of the DM was measured by the 2D normalized pixel error, with lower numbers indicating better reconstruction performance. We first acquired the Euclidean distance between the landmarks in DM-reconstructed videos and the corresponding landmarks in original videos (Fig. 2a). The pixel errors between landmarks were then normalized by the pixel distance between the centers of the two eyes.
For the eyes of 405 patients, the average normalized pixel errors in images of patients with TAO, strabismus, ptosis and nystagmus were 0.85%, 0.81%, 0.82% and 1.00%, respectively, in eyeball reconstruction and 1.52%, 1.24%, 1.52% and 1.61%, respectively, in eyelid reconstruction (Fig. 2b). The heat map of the normalized pixel errors in images of patients with the abovementioned four diseases is shown in Fig. 2c. The normalized pixel errors remained small and stable most of the time, with slight fluctuations when the eyes were looking down, thus indicating the precise reconstruction of the DM.
Clinical validation of DM
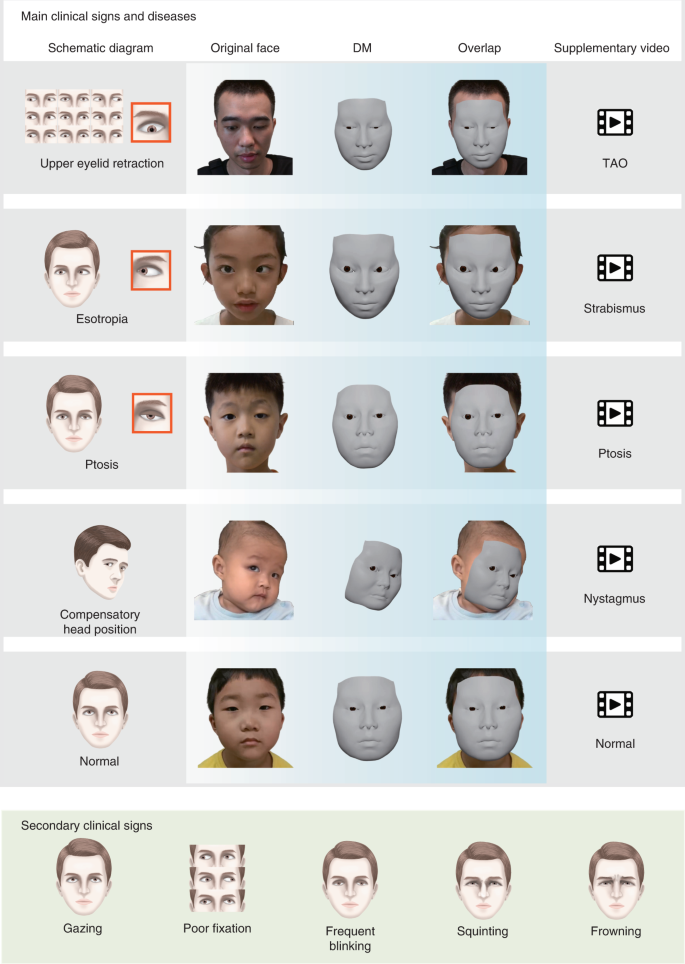
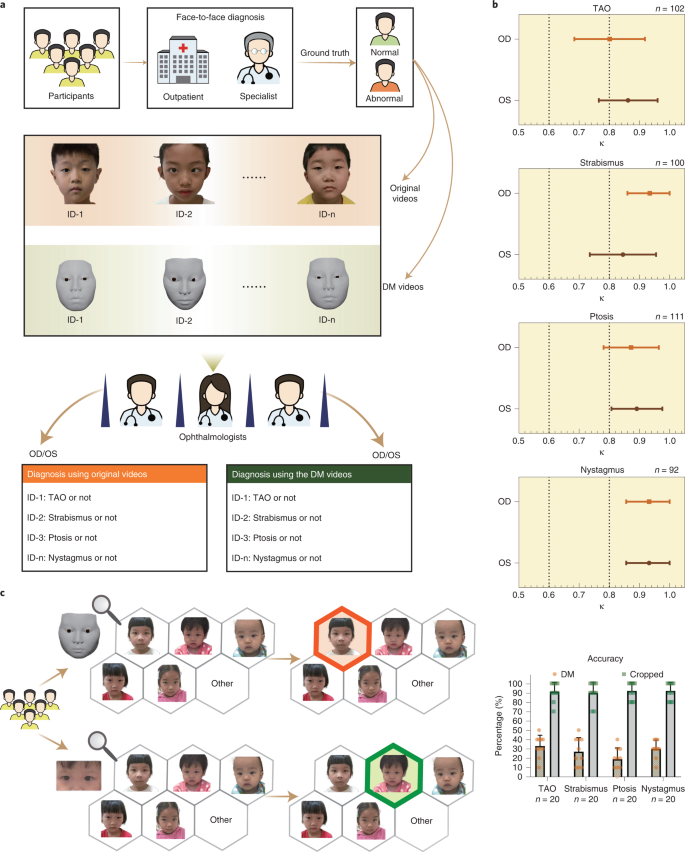
To evaluate the performance of the DM in clinical practice, we performed a relevant diagnostic comparison and an identity-removal validation. In the relevant diagnostic comparison, 12 ophthalmologists, 3 from each of the four departments, were invited to diagnose patients from their departments based on the DM-reconstructed videos and original videos. We evaluated the videos regarding pathological ocular manifestations that caused changes in the appearance of the eye and patients were diagnosed visually with diseases, including (1) TAO (exophthalmos, eyelid retraction and overactive or underactive extraocular muscles); (2) strabismus (horizontal or vertical strabismus and compensatory head position); (3) ptosis (drooping or lowering of the upper eyelid); and (4) nystagmus (Fig. 3 and Supplementary Video)9. For each eye, both the independent diagnosis from the original videos and the diagnosis from the DM-reconstructed videos were recorded and compared (Fig. 4a and Supplementary Data 1). If the two diagnoses were excellently consistent, this would suggest that the reconstruction was precise enough for use in clinical practice. Cohen’s κ values showed very high consistency (κ = 0.845–0.934 for strabismus, ptosis and nystagmus on both eyes and κ = 0.801 for TAO on right eyes) of the diagnoses, made by three ophthalmologists under majority rule, from original and reconstructed videos for all comparisons (Fig. 4b and Extended Data Table 2). Additionally, the accuracies of the diagnoses from the original and reconstructed videos, compared to the ground truth, were comparable for all paired comparisons (P = 0.131–1; Extended Data Table 3). These results indicate that the DM retains the important clinical attributes correctly and has the potential to be adopted in clinical practice.
In the identity-removal validation, we compared the identity-removal ability of the DM with
that of cropping by using multiple-choice questions. Specifically, we processed the original images of the faces of the patients by using DM and cropping to generate 400 DM-reconstructed images and 400 cropped images, respectively. The selected generated images and the original images were staggered in the video time sequence. Correspondingly, we designed 800 multiple-choice questions. For the DM test, each question contained a DM-reconstructed image and five original images. For the cropping test, each question contained a cropped image and five original images. For each question, there were six options, including the five original images and an ‘other’ option. From these options, the respondents were asked to find the original image corresponding to the DM-reconstructed image or cropped image. The results showed that the accuracy rate for those taking the DM test was 27.3%; however, the accuracy for those taking the cropping was 91.3%, which was much greater than the accuracy of those taking the DM test (Fig. 4c). Both accuracies were likely overestimated because the test was conducted on the premise that the respondent knew only five people. In actual situations, the numbers of people are far higher; however, the results still demonstrate that the DM can effectively remove patient identity attributes and protect patient privacy, especially compared to cropping.
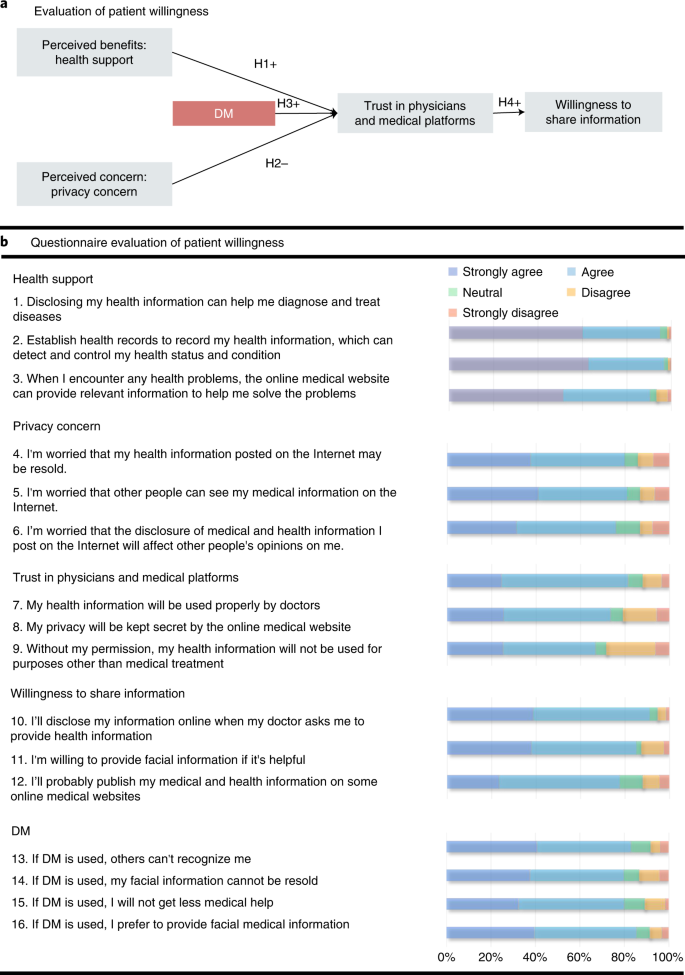
In addition, to evaluate the willingness of patients to share their eye and facial images during the application of DM, we performed an empirical investigation. 3D reconstruction software was developed to which users could provide their videos anonymously. The videos were then automatically processed by the DM and delivered to clinicians. Clinicians were only allowed to watch the DM-reconstructed videos for diagnosis (Supplementary Video) and the diagnoses were fed back to the users. A total of 317 outpatients, randomly selected via clinics, agreed to participate in the empirical investigation. During the investigation, the participants were asked to watch uploaded videos and the corresponding reconstructed videos processed by the DM using the software. The patients then completed a questionnaire to investigate their willingness to use DM at the end of the investigation (Fig. 5a). Among the respondents, 161 were males (50.7%). By age group, the highest proportion of respondents was in the 20–30-year group. Most of the respondents had university degrees (82.3%) and had used smartphones for more than 7 years (73.8%). In addition, in the questionnaire, regarding five hypotheses, 16 questions were designed from five aspects, including health support, privacy concerns, trust in physicians and medical platforms, willingness to share information and the influences of DM (Fig. 5b). The Kaiser–Meyer–Olkin measure of sampling adequacy and Cronbach α values for each component were larger than 0.617 and 0.718, respectively, thus supporting the reliability and validity of each question in the research design. Approximately 80% of the participants agreed that they had privacy concerns. Among the participants who had a disease with facial signs, more than 81.4% had privacy concerns, compared to more than 74.4% of participants without facial signs. Furthermore, we assessed the significance of the influence of the major aspects.
As shown in Extended Data Table 4, perceived benefits, such as health support of digital health information, positively affected patients’ trust in physicians and medical platforms with respect to digital health (β = 0.465, P < 0.001). In contrast, perceived concerns, such as privacy concerns, negatively affected patients’ trust in physicians with respect to digital health (β = −0.158, P = 0.005). The hypothesis that the DM had a positive impact on such trust was supported (β = 0.348, P < 0.001), thereby further improved the patients’ willingness to share information (β = 0.503, P < 0.001). The questionnaire details of each patient are included in Supplementary Data 2.
AI-powered re-identification validation of the DM
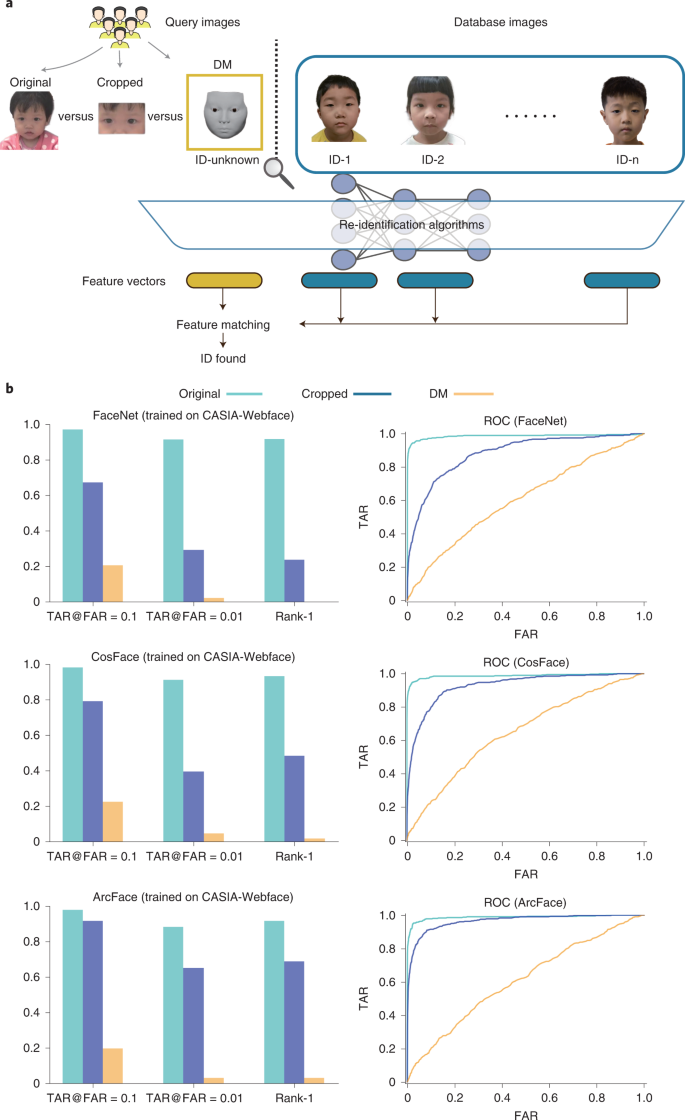
To evaluate the performance of the DM in evading recognition systems, we performed an AI-powered reidentification validation (Fig. 6a). In the validation, we conducted face recognition attacks by using three well-known deep-learning systems, namely, FaceNet20, CosFace21 and ArcFace22. All the systems were trained on the CASIA-WebFace Dataset23, which contains 494,414 face images of 10,575 real identities collected from the web. Using 405 patient videos, we randomly selected two frames in each video; one of the frames was used as the query image and the other was used as the database image. We processed 405 original query images to further generate 405 cropped query images and 405 DM-reconstructed query images. For the test, given a query image (original images, cropped images or DM-reconstructed images), the face recognition system (FaceNet, CosFace or ArcFace) was asked to match the image with database images of 405 patients. We used the area under the receiver operating characteristic curve (AUC), TAR@FAR = 0.1 (TAR, true accept rate; FAR, false accept rate), TAR@FAR = 0.01 and Rank-1 to evaluate the face recognition performance. The lower values of TAR@FAR = 0.1, TAR@FAR = 0.01 and Rank-1 indicate the weaker performance of the face recognition system and the greater performance of the privacy protection technology. As shown in Fig. 6b and Extended Data Table 5, the results on all the measurements show that taking original images as the query images, it was easy for face recognition systems to match the correct identity. When taking cropped images as the query images, the metrics had limited degradation. When using the DM, the performance of face recognition was significantly degraded. Rank-1 was <0.02 for all three systems, indicating that the systems had a very low possibility of identifying the correct identity with the DM-reconstructed images. Meanwhile, the receiver operating characteristic (ROC) curves of using DM-reconstructed images were close to y = x for all three systems, indicating that it was impossible to keep high TAR with low FAR. These results show the superiority in terms of privacy protection of our DM technique.
Discussion
In this study, we developed and validated a new technology called DM, which is based on real-time 3D reconstruction and deep learning, to retain the clinical attributes contained in patient videos, while minimizing access to nonessential biometric information for added personal privacy in clinical practice. Experimental results support that with the DM, examination videos of patients with manifestations of ocular disease can be precisely reconstructed from 2D videos containing original faces. A clinical diagnosis comparison showed that ophthalmologists achieved high consistency in reaching the same diagnosis when using the original videos and the corresponding DM-reconstructed videos. This new technology could effectively remove identity attributes and was positively accepted by patients with ocular diseases, who expressed an increasing willingness to share their personal information and have it stored digitally with this added layer of biometric protection.
It is notable that the DM offers a pragmatic approach to safeguarding patient privacy and data utilization in both research and clinical settings; patient privacy and data utilization are frequently cited as concerns by patients worried about data breaches. Compared to rather crude but still widely used options, such as covering identifiable areas with very large bars or cropping these areas out altogether3, the DM is a much more sophisticated tool for anonymizing facial images. Even next-generation privacy-protection techniques, such as federated learning and homomorphic encryption, do not safeguard privacy well and crucially, these techniques are vulnerable to model inversion or reconstruction attacks24. The DM selects relevant features for reconstruction, but it is impossible to reconstruct original data particularly relevant to patient identification. Furthermore, compared with other face-swapping technologies, the DM can obtain quantitative parameters (such as the degree of eyeball rotation, eyelid shape parameters, blinking rate and rotation frequency), which might prove essential in the future for intelligent diagnosing disease or studying the relationships between diseases and certain facial characteristics.
In addition to its potential utilization in research and routine clinical practice, the DM can be applied to telemedicine, including online automatic diagnosis and patient triage for more efficient healthcare delivery25. The wider adoption of digital medicine, partly prompted by the ongoing COVID-19 pandemic, will require that the barriers to privacy protection be overcome and an important step is removing biometric data that are not essential for healthcare delivery. The DM can encrypt data before they are submitted to the cloud, thereby allowing clinicians or AI algorithms to review the reconstructed data and removing concerns of patients whose medical records contain sensitive biometric data26.
However, ‘protecting privacy’ does not equate to ‘absolute removal of identity characteristics.’ According to the Health Insurance Portability and Accountability Act Privacy Rule, protecting patient privacy refers to reducing the identification risk of health information27. One of the most important principles is balancing disclosure risk against data utility. Therefore, the purpose of this study is to provide an approach to health information disclosure that de-identifies protected health information as much as possible, without compromising the need for the clinician to reach a diagnosis.
The study has several limitations. First, the reconstruction of conjunctival hyperemia, eyelid edema and abnormal growth of tissues, such as ocular tumors, remains challenging because of insufficient model capacity. Model-based 3D reconstruction assumes that the target lies in the linear space spanned based on a set of prepared models; however, it is difficult to cover all shapes in the aforementioned cases because shapes differ significantly from person to person. We intend to improve the DM by including a sufficiently large sample of abnormal cases for more detailed analysis or constructing an extra submodel on top of the existing model in the next research step. Second, this paper has demonstrated that the DM can protect re-identification from images but it may not work under certain circumstances if the video of the patient is exposed. We are currently extending our work to deal with video protection and circumvent this possible weakness. Third, the potential risk that the DM might be attacked still remains, as it might be abused to develop targeted attack algorithms; however, this risk can be mitigated by formulating relevant rules in the future.
In conclusion, we demonstrate the effectiveness of the DM in enhancing patient data privacy by making use of deep learning and real-time 3D reconstruction and notably, we demonstrate the DM’s acceptability to healthcare users. Future work is necessary to further evaluate the applicability of DM in a wider variety of clinical settings as the requirements for de-identification will vary according to the type of imaging dataset used.
Methods
Ethical approval
The research protocol and ethical review of this study were approved by the Institutional Review Board/Ethics Committee of the Zhongshan Ophthalmic Center. The clinical study protocol is shown in the Supplementary Note. Consent was obtained from all individuals whose images are shown in figures or the video for publication of these images. Informed consent was obtained from at least one legal guardian of each infant and the tenets of the Declaration of Helsinki were followed throughout this study. The trial in this study was registered with the Clinical Research Internal Management System of Zhongshan Ophthalmic Center and retrospectively registered at ClinicalTrials.gov (NCT05058599).
DM technique
Our reconstruction method consisted of three main stages: face reconstruction, eyelid reconstruction and eyeball reconstruction. At each stage, a unique detector was used to extract relevant features (the face landmarks Lface at the first stage, the eyelid landmarks Leyelid and eyelid semantic lines Seyelid at the second stage and the iris landmarks Liris at the last stage). All detectors were neural networks based on deep-learning techniques. After acquiring the features, the corresponding model parameters were optimized to fit these features. The details of each stage are described below.
Face reconstruction
As we use a bilinear model17, we generated a 3D face Mf∈R3NF using a shape vector wfs∈RNfs and a set of motion vectors wfm∈RNfm:
Mf=Cr×2wfs×3wfm
(1)
where Cr∈R3NF×Nfs×Nfm is a pre-defined core tensor that stores 3D vertex positions of faces covering the major variations in shape and motion; ×2 and ×3 are the tensor product operations on the second dimension and third dimension, respectively; NF is the number of 3D face vertices; and Nfs and Nfm are the dimensions of the shape vector and motion vector, respectively.
Given the face landmarks Lface∈R2NLF on a video frame of a patient, we reconstructed a 3D face of the patient by solving an optimization problem; minimizing the landmark registration error Eface by searching for the optimal parameters wfs, wfm, R and t:
Eface(wfs,wfm,R,t;Cr,Lface)=∑iNLF∥∥Lfacei−Π(Mf′cface(i))∥∥22
(2)
where R ∈ SO(3) and t∈R3 denote the rotation and translation of a 3D mesh, respectively; ∏(·) is a projection function that projects 3D points to 2D points; cface(i) represents the corresponding face index for the ith face landmark, which is predefined manually; and NLF is the number of face landmarks.
Note that wfs was estimated based on only the first frame and then fixed for the following frames. Therefore, for the following frames, the objective function was slightly simplified to
Eface(wfm,R,t;wfs,Cr,Lface)=∑iNLF∥∥Lfacei−Π(Mf′cface(i))∥∥22
(4)
Eyelid reconstruction
Similar to the bilinear face model, our eyelid model18 contained a set of shape vectors wes∈RNes and a set of motion vectors wem∈RNem. Nes and Nem are the dimensions of the shape vector and motion vector, respectively. Given our parametric eyelid model, a particular 3D eye region Me∈R3ND was reconstructed as follows:
Me=Me0+∑iNeswesiMesi+∑jNemwemjMemj
(5)
where Me0∈R3ND is the template eyelid geometry model; Mes∈RNes×3ND and Mem∈RNem×3ND are also predefined and represent the basis geometry changes for shape and motion, respectively; and ND is the number of 3D eyelid vertices.
Before reconstruction, we first fitted two polynomial curves for the upper eyelid and the lower eyelid according to the detected landmarks Leyelid∈R2NLd. Specifically, we fit cubic polynomial curves:
y=ax3+bx2+cx+d
(6)
by solving a least-squares problem:
⎛⎝⎜⎜⎜⎜⎜11⋮1x1x2⋮xNLdx21x22⋮x2NLdx31x32⋮x3NLd⎞⎠⎟⎟⎟⎟⎟⎛⎝⎜⎜⎜dcba⎞⎠⎟⎟⎟=⎛⎝⎜⎜⎜⎜y1y2⋮yNLd⎞⎠⎟⎟⎟⎟
(7)
x and y denote the 2D coordinates of a point on 2D image. Then, we applied dense sampling to acquire dense landmarks Ldense∈R2NLD by uniform sampling xdense={xdense1,xdense2,⋯,xdenseNLD}.
Ldensei=(xdensei,ydensei)=(xdensei,a(xdensei)3+b(xdensei)2+c(xdensei)+d)
(8)
where NLd is the number of detected eyelid landmarks and NLD is the number of dense landmarks.
For continuous features, the four detected semantic lines (representing the double-fold, the upper eyelid, the lower eyelid and the lower boundary of the bulge) Seyelid are irregular curves defined on the 2D image space, thus indicating the positions of different parts of the eyelid.
Integrating both discrete features and continuous features, we solved the following energy function to search for the optimal wes and wem:
Eeyelid(wes,wem;R,t,Me0,Mes,Mem,Ldense)=∑iNLD∥∥Ldensei−Π(Me′ceyelid(i))∥∥22
(9)
Esl(wes,wem;R,t,Me0,Mes,Mem,Seyelid)=∑kNsl∑j∈vsl(k)dis(Π(Me′j),Seyelidk)2
(10)
where ceyelid(i) represents the corresponding vertex index for the ith eyelid landmark, which is also manually predefined in advance. vsl(k) represents a set of vertex indices belonging to the kth semantic line. dis(·,·) is the distance between a point and the closest point on a line. Nsl is the number of semantic lines, which is four in this paper. R and t are calculated at the face reconstruction stage.
Similar to the face reconstruction, wes was determined in the first frame. In the following frames, the objective functions were changed to
Eeyelid(wem;wes,R,t,Me0,Mes,Mem,Ldense)=∑iNLD∥∥Ldensei−Π(Me′ceyelid(i))∥∥22
(12)
Esl(wem;wes,R,t,Me0,Mes,Mem,Seyelid)=∑kNsl∑j∈vsl(k)dis(Π(Me′j),Seyelidk)2
(13)
Eyeball reconstruction
Our SGAEM model19 represented a 3D eyeball B∈R3NB based on the eyeball radius re and the iris radius ri. NB is the number of 3D eyeball vertices.
B=SGAEM(re,ri)
(14)
The position of the eyeball relative to the face pe∈R3 is also needed to be determined for reconstruction. Here, certain prior knowledge is used to estimate the three parameters (re, ri and pe) in the first frame and then fixed in the following frames by minimizing the following objective function:
Eeyeball(θ,ϕ;R,t,Liris,re,ri,pe)=∑iNLB∥∥Lirisi−Π(B′ciris(i))∥∥22
(15)
B′=R(Rot(θ,ϕ)B+pe)+t
(16)
where θ and ϕ are the Euler angles of eyeball rotation. Rot(·,·) is a function that converts θ and ϕ into a rotation matrix. ciris(i) represents the corresponding vertex index for the ith iris landmark, which is also predefined in advance. NLB is the number of iris landmarks. R and t are calculated at the face reconstruction stage.
Sequence consistency
To maintain consistency between successive frames, the following smoothing terms were also considered for the above objective functions:
Esmooth1=λfm∥∥wfm−wfmprev∥∥22+λR∥R−Rprev∥22+λt∥t−tprev∥22
(17)
Esmooth2=λem∥wem−wemprev∥22
(18)
Esmooth3=λθ∥θ−θprev∥22+λϕ∥ϕ−ϕprev∥22
(19)
where subscript prev represents the parameter at the previous frame.
Finally, the objective function for the three stages becomes
E1=Eface+Esmooth1
(20)
E2=Eeyelid+Esl+Esmooth2
(21)
E3=Eeyeball+Esmooth3
(22)
The Gauss–Newton method was adopted to solve the nonlinear least-squares problem to minimize each objective function.
Network training
We introduced how to train the networks of the three landmark detectors (face, eyelid and iris landmark detectors) and the eyelid semantic line detector (Supplementary Table 1). The network architecture for the three landmark detectors follows HRNet28. For the face detector, we used both the 300W29 and WFLW30 to train the network. For the eyelid and iris landmark detectors, we used UnityEyes31 to synthesize 20,000 images with groundtruth landmark positions in the training. The network architecture for the eyelid semantic line detector follows HED32 and we used the data in our previous work18 to train it. After all these networks were trained, we further used our own collected 775 patient portraits to fine-tune the networks, making the networks better able to handle the data of real patients. Specifically, we split 75 patient portraits from the fine-tuning dataset for validation and used the remaining 700 portraits for fine-tuning. The characteristics of the training dataset are shown in Extended Data Table 6.
Deformation transfer for eyebrow movements
Although the linear eyelid model provided sufficient eyelid variations, it included no degrees of freedom for motion in the eyebrow region. Reconstructing the eyebrow motions of patients would help in diagnosing TAO. To perform such a reconstruction, a deformation transfer method was applied, as described below.
We defined two semantic regions on both the face and eyelid models and by assuming that the region on the face model influences the corresponding semantic region on the eyelid model, the influence of the face vertex on the eyelid vertex could be estimated based on the influence weights wi,j:
wi,j=exp(−∥∥vfi−vej∥∥222r2)
(23)
where vfi and vej represent the ith face vertex and jth eyelid vertex, respectively and r is the influence radius. With wi,j, each eyelid vertex vej can be deformed together with the motion of the face vertices as follows:
vej,t=vej,t−1+∑i∈N(j)wi,jN(vfi,t−vfi,t−1)
(24)
where t and t − 1 represent the time index of the current frame and the previous frame, respectively. N(j) is a set of face vertex indices related to vej and N is the number of related vertices. Notice that all the vertices were in the local coordinate system, which removes the influence of global rotation R and global translation t.
Definitions of pathological ocular manifestations for the clinical evaluation
Ptosis is defined as the upper eyelid falling to a position that is lower than normal (typically 1.0–2.0 mm below the superior corneoscleral limbus)33. The palpebral fissure distance is often evaluated by guiding the patient’s eye fixation to a distant target34. The frontalis muscle, levator palpebrae muscle and orbicular muscle are analyzed based on a series of movement guidelines to preliminarily explore the cause of ptosis; these movement guidelines include having the patient gaze upwards and downwards, maintain an upwards gaze for 1 min and close his or her eyes tightly shut35. Additionally, the presence of Brown’s ocular movements and jaw motion are all provided to aid in diagnosing ptosis36.
Strabismus is characterized as the eyes not properly aligning with each other when looking at an object. The cover test and alternate cover test are used in diagnosing strabismus37. Because most people have exotropia but do not need treatment, we excluded exotropia when determining the diagnosis of strabismus. The test allows wearing glasses, especially in the case of patients with accommodative esotropia.
TAO is diagnosed by positive responses of eyelid retraction and at least two of the following four sets of findings: chemosis or eyelid edema, lid lag or restrictive lagophthalmos38,39.
Nystagmus is characterized as the eyes moving rapidly and uncontrollably; this movement can be observed and diagnosed during eye movement recording40. Additionally, compensatory head position and median zone are important features of nystagmus41.
Statistical analysis
In the sample size estimate of the clinical trial, the power was set at 0.9, the significance level was 0.025 and a one-sided test was used. Assuming k1 = 0.85 and k0 = 0.6, the probabilities of abnormal findings were 0.3 to 0.7 and the sample size for each disease was at least 82 estimated using the irr package in R 4.1.1 (R Project for Statistical Computing).
Our quantitative evaluation was based on the 2D pixel distance between the detected 2D landmarks and projected 2D positions of the 3D points on the reconstructed face. To exclude the influence of face size, we evaluated our method using the normalized pixel distance rather than the absolute pixel distance.
To acquire the normalized pixel distance, we calculated the absolute pixel distance first:
Dabsi=∥Li−Π(Vi)∥2
(25)
where Li is the ith 2D landmark and Vi is the ith 3D point.
Then, we calculated the absolute pixel distance between the two eyes:
Deye=∥∥Cleft−Cright∥∥2
(26)
where Cleft and Cright are the center positions of the left and right eyes, respectively.
Finally, we normalized the pixel distance between landmarks according to the distance between the two eyes, that is,
Dnormi=DabsiDeye
(27)
To further validate the reconstruction, the maximum normalized error and average normalized error were defined as
MRE=max{Dnorm1,Dnorm2,…,DnormN}
(28)
ARE=∑Ni=1DnormiN
(29)
Generally, n = 38 for eyeball validation and n = 200 for eyelid validation, but we excluded some (landmark, point) pairs when they were occluded, especially in the strabismus dataset.
In the clinical validation, the characteristics of the participants were described as the frequency (proportion) for categorical variables and the median (IQR) for continuous variables due to nonnormal distributions. Cohen’s κ statistics were used to evaluate the diagnostic consistency in the relevant diagnostic comparison. Kappa was interpreted as recommended by Landis and Koch, where κ ≤ 0.00 is considered as poor, 0.00–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial and ≥0.81 almost perfect42. In addition, based on the groundtruth, we measured the accuracies of diagnoses from the original videos and diagnoses from the reconstructed videos and compared them using the McNemar test. In the empirical investigation, principal-component analysis was used to generate five factors from the 16 questions in the questionnaire. The Kaiser–Meyer–Olkin measure of the sampling adequacy and Cronbach’s α for each component were used to evaluate the reliability and validity of each question. Linear regression was used to measure the associations between components.
In the AI-powered re-identification validation, we used AUC, TAR@FAR = 0.1, TAR@FAR = 0.01 and Rank-1 to evaluate the performance of face-recognition systems. The TAR is the proportion of authorized people who the system correctly accepts and is defined as
The FAR is the proportion that the system incorrectly accepts nonauthorized people, defined as
By setting different threshold values for similarity scores (given by the face recognition systems), we obtain different TARs and FARs, resulting in a ROC curve. The AUC measures the 2D area underneath the ROC curve. TAR@FAR = X represents the TAR value when FAR equals X. Rank-1 is the probability that the similarity score of the same identity ranks first among all the identities.
Data were analyzed using SPSS (v.23.0, IBM Corp), R (v.4.1.1, R Project for Statistical Computing), C++ (v.11, Standard C++ Foundation) and Python (v.3.6, Python Software Foundation) with a designated significance level of 5%.
Algorithm efficiency
Although considerable engineering effort is still needed to build a practical application, our main algorithm can run in real time. In detail, our algorithm takes approximately 7 ms, 14 ms and 4 ms per frame for face, eyelid and eyeball reconstruction, respectively, on one Intel i7 CPU and one NVIDIA 1080 GPU.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.